Reviewed by Danielle Ellis, B.Sc.Oct 5 2022
Metastasis, the process through which cancer spreads and develops new tumors, is to account for about 90% of cancer-related fatalities. The 5-year survival rate following surgery to remove liver metastases is as low as 30–50%, making the development of treatments to prevent liver metastasis urgently required. The liver is thought to be the organ most susceptible to metastatic malignancy.
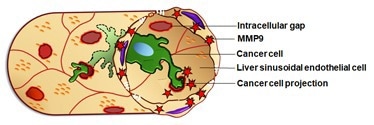 The liver sinusoidal endothelial cells (LSECs, beige) surround a blood vessel containing a cancer cell (green). The cancer cell is able to induce the formation of intracellular gaps (iGaps, red), through inducing MMP9. Image Credit: Osaka Metropolitan University
The liver sinusoidal endothelial cells (LSECs, beige) surround a blood vessel containing a cancer cell (green). The cancer cell is able to induce the formation of intracellular gaps (iGaps, red), through inducing MMP9. Image Credit: Osaka Metropolitan University
The alternative pathway for liver metastasis has been revealed by a team of researchers, including graduate student Truong Huu Hoang, Professor Norifumi Kadawa from the Graduate School of Medicine at Osaka Metropolitan University, and Associate Professor Misako Matsubara from the Graduate School of Veterinary Science.
They have also clarified the molecular mechanism at play. Their findings are anticipated to culminate in the creation of medications to both prevent and treat metastatic liver cancer.
Image Credit: Christoph Burgstedt/Shutterstock.com
Although it is known that metastatic cancer cells alter the microenvironment of liver cells in ways that encourage metastasis, the entire scope of these interactions has not yet been extensively explored.
Liver sinusoidal endothelial cells (LSECs), which border the blood arteries of the liver to create a protective barrier, come into touch with cancer cells carried in the bloodstream.
The liver’s detoxifying processes are carried out by LSECs, which have a large number of small pores through which only minute particles and liquid blood components—but not cancer cells—can pass.
Under stressful circumstances, LSECs are continuously exposed to hazardous compounds delivered by the blood that can disrupt these tiny pores, resulting in the formation of bigger intracellular gaps and a weakened protective barrier. This prompted the study team to wonder whether the intracellular gaps in LSECs play a role in liver metastasis.
By injecting cancer cells into the spleen to produce a mouse model of liver metastasis, the study team examined alterations in the LSECs using an omics analysis.
They discovered that the LSECs produced a number of proteins as a result of the cancer cells’ movement from the spleen to the liver. Matrix metalloproteinase 9 (MMP9), one of these proteins, was expressed in LSECs and resulted in the formation of intracellular gaps.
Furthermore, the researchers demonstrated that cancer cells expanded their projections directly into the intracellular gaps of LSECs, allowing them to penetrate the liver tissue. This was accomplished using electron microscopy and 3D tomography reconstruction.
They discovered a direct link between the number of intracellular gaps in the LSECs and the number of newly developed metastatic liver cancers in the mice. However, administering an MMP9 inhibitor to the mice stopped the development of new tumors, indicating that MMP9 represents a possible therapeutic target to inhibit liver metastasis.
In this study, we discovered a new phenomenon related to metastasis: cancer cells induce LSEC intracellular gap formation and infiltrate the liver through those gaps. With these results we are continuing our research to develop new treatments for liver metastasis, targeting intracellular gap formation in LSECs.”
Misako Matsubara, Associate Professor, Graduate School of Veterinary Science, Osaka Metropolitan University
Source:
Journal reference:
Hoang, T. H., et al. (2022). Cancer cells produce liver metastasis via gap formation in sinusoidal endothelial cells through proinflammatory paracrine mechanisms. Science Advances. doi.org/10.1126/sciadv.abo5525