Membrane proteins are vital therapeutic targets. They exist between the interior and exterior of human cells. Some of them, known as “transporters,” transport substances into and out of the cellular environment. However, removing and retaining them for observation is a very difficult task.
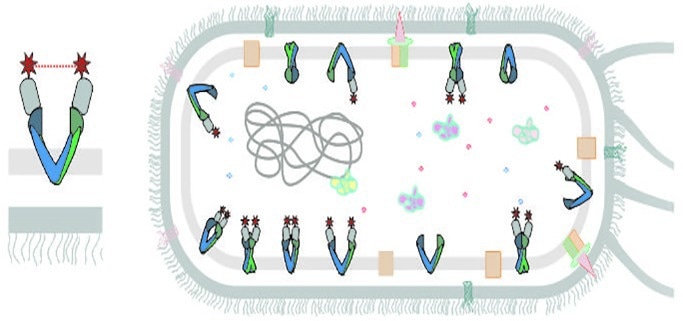 Nanobodies (grey) with magnetic probes (red stars) target the desired membrane protein. Image Credit: © Enrica Bordignon
Nanobodies (grey) with magnetic probes (red stars) target the desired membrane protein. Image Credit: © Enrica Bordignon
In partnership with the University of Zurich (UZH), a team from the University of Geneva (UNIGE) has devised an innovative method for studying their structure in their natural environment: the cell. The method relies on electron spin resonance spectroscopy. These findings, which were recently published in the journal Science Advances, might aid in the creation of novel drugs in the future.
Each cell in a living organism is enclosed by a cell membrane (or “cytoplasmic membrane”). This membrane is made up of two layers of lipids. It divides the cell’s contents from its immediate surroundings and controls the substances that can either enter or leave the cell. Membrane proteins are proteins that are connected to this membrane.
They are situated at the interface between the outside and inside of the cell and transport different substances across the membrane—into or out of the cell—and play an important role in cell signaling, which is the communication system that enables cells to synchronize their metabolic processes, development, and organization. As a result, membrane proteins account for more than 60% of currently available drug targets.
Difficult objects to study
The biophysical investigation of their structure and the constituent amino acids' spatial organization is critical. Researchers must retrieve these proteins from the cell membrane where they are present and isolate them from all other proteins to describe them.
Membrane proteins, once removed, cannot be examined in aqueous solutions. They must be kept in detergent-based liquid solutions. They can also be put into “nanodiscs,” which are artificial membranes consisting of proteins and lipids, or into pure lipidic membranes.
These methods, in any case, take them out of their natural environments and prevent close in situ examination of how they function. Proteins may exhibit distinct structural features when removed from their usual environment, affecting drug development.
A revolutionary method
Enrica Bordignon, full professor in the Department of Physical Chemistry at the UNIGE Faculty of Science, headed a team along with Markus A. Seeger, associate professor at the UZH’s Institute for Medical Microbiology, have invented a new technique for analyzing membrane proteins in action in living cells; specifically, the inner cell membranes of the intestinal bacterium E. coli. The study team depended on a unique “tool” to accomplish this: nanobodies.
These are fragments of antibodies that can recognize and bind to a specific target, such as an antigen or, in our case, a membrane transporter, in a very efficient way.”
Enrica Bordignon, Full Professor, Department of Physical Chemistry, University of Geneva
Thus, the experts created unique nanobodies for a membrane transporter and directly used them to report on its structure.
Inserted into E. coli cells, two nanobodies target the desired membrane protein on the inner membrane of the cell and attach to it.”
Markus A. Seeger, Associate Professor, Institute for Medical Microbiology, University of Zurich
Researchers from Ruhr University Bochum (RESOLV cluster of excellence), the University of Osnabrueck, Germany, and the University of Southampton, UK, were also part of the multidisciplinary group.
New targets for certain drugs
A tiny magnetic probe (a molecule bearing unpaired electrons) was previously linked to each nanobody.
When two nanobodies bind to the transporter, we can measure the distance between the two magnetic probes in cells using our EPR methods.”
Enrica Bordignon, Full Professor, Department of Physical Chemistry, University of Geneva
This method is known as “electron paramagnetic resonance spectroscopy” (EPR) or “electron spin resonance.” The measured distance is in the nanometer range (one-millionth of a millimeter).
Enrica Bordignon adds, “For the first time, we have managed to obtain a clear picture of the conformation of a membrane protein in its real environment, and we could follow the change induced when we modified one single amino acid into another one.”
“The development of this new strategy results from excellent and challenging teamwork between our two groups at UNIGE and UZH. In particular, it is the resilience of the two first authors, Dr. Laura Galazzo (UNIGE) and Dr. Gianmarco Meier (UZH), that made this project a success after five years of research,” emphasized the researcher.
This novel method effectively evaluates membrane protein characteristics in their natural environment. It allows for a better knowledge of how these proteins transport things into and out of the cell.
This approach has the additional benefit of being readily transposable to mammalian cells. It could then be utilized to better understand and target the membrane proteins that reject specific anti-cancer drugs outside the cell, thereby combating the phenomena of multi-drug resistance.
Source:
Journal reference:
Galazzo, L., et al. (2022) The ABC transporter MsbA adopts the wide inward-open conformation in E. coli cells. Science Advances. doi.org/10.1126/sciadv.abn6845.