A novel method of reprogramming the immune cells to shrink or destroy cancer cells has been demonstrated to work in melanoma, a difficult to treat and destructive skin cancer. The study, conducted by the University of Bristol, was published on October 31st, 2022, in the Advanced Science journal and offers a new approach to eliminate early stage pre-cancerous and even late-stage tumor cells.
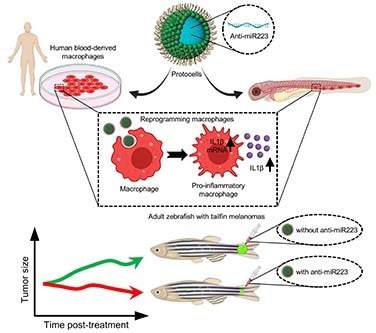 Illustration showing how miniature artificial protocells loaded with anti-microRNA-223 cargo can reprogram cancer-associated macrophages in larval and adult zebrafish leading them to be more pro-inflammatory and thus able to drive melanoma shrinkage. Image Credit: Paco Lopez Cuevas
Illustration showing how miniature artificial protocells loaded with anti-microRNA-223 cargo can reprogram cancer-associated macrophages in larval and adult zebrafish leading them to be more pro-inflammatory and thus able to drive melanoma shrinkage. Image Credit: Paco Lopez Cuevas
The researchers were able to convert these cells into a state that makes them more effective at slowing down the growth and killing of melanoma cells by using miniature artificial capsules termed protocells crafted to deploy reprogramming cargoes that are taken up by inflammatory cells (white blood cells). They demonstrated that it was possible for both animal and human immune cells to do so.
The research is the first to assess a protocell's capacity to deliver cargo for immune cell reprogramming, and it provides a potential new target for the development of cancer immunotherapies.
One of the study’s lead authors, Paul Martin, Professor of Cell Biology at the School of Biochemistry at the University of Bristol, detailed what transpires when the immune system is confronted with cancer cells.
Our immune cells have a surveillance capacity which enables them to detect pre-cancerous cells arising at any tissue site in the body. However, when immune cells encounter cancer cells they are often subverted by the cancer cells and instead tend to nourish them and encourage cancer progression. We wanted to test whether it might be possible to reprogramme our immune system to kill these cells rather than nurture them.”
Paul Martin, Study Lead Author and Professor, Cell Biology, School of Biochemistry, University of Bristol
The scientists first tested the proof of concept in zebrafish larvae. The translucency allows investigators to see inflammatory immune cells interacting with cancer cells in ways that are not possible within human tissues.
Protocells equipped with anti-miR223 molecules, which bind to and interfere with signaling machinery in inflammatory immune cells, efficaciously extending their pro-inflammatory state, were shown to initiate altered immune cell-cancer cell interactions, delaying cancerous cell growth and increased tumor cell death in larvae.
The experiment was replicated in adult fish with tailfin melanomas to see if this approach could be scaled up as a viable treatment strategy for shrinking larger, more established, and developing tumors, and it showed that this approach significantly suppressed melanoma cell growth.
To thoroughly evaluate the viability of employing protocells to deliver “reprogramming” anti-miR223 cargoes in individuals, the experiment was repeated in vitro with primary human immune cells from the Toye lab, also at Bristol’s School of Biochemistry. The results of this experiment showed that the protocells could effectively transfer and reprogram human immune cells into a more durable pro-inflammatory and presumably anti-cancer state.
Our results highlight the therapeutic benefits of harnessing host immunity to eradicate cancers and demonstrate the feasibility of using protocells to deliver cargoes for reprogramming innate immune cells. While our experiments in zebrafish are early pre-clinical studies, our results indicate that the same is possible for human immune cells, at least in vitro, and can be similarly reprogrammed to suppress cancer growth.”
Stephen Mann, Professor, School of Chemistry, University of Bristol
Stephen Mann is also associated with the Max Planck Bristol Centre for Minimal Biology.
Source:
Journal reference:
López-Cuevas, P., et al. (2022) Macrophage Reprogramming with Anti-miR223-Loaded Artificial Protocells Enhances In Vivo Cancer Therapeutic Potential. Advanced Science. doi.org/10.1002/advs.202202717.