Researchers of RIKEN identified the structure of the “antenna” that a blue-green alga employs to yield light and compared it with those of four other species. The study can throw light on the development of potential photoreactive compounds in addition to offering clues about the diversity and evolution of cyanobacteria.
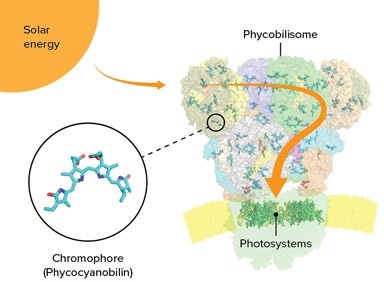 Chromophores interspersed in the phycobilisome of cyanobacteria act as tiny antennas, capturing sunlight. The energy is then conveyed to photosystems at the base of the phycobilisome. Image credits: © 2022 RIKEN SPring-8 Center
Chromophores interspersed in the phycobilisome of cyanobacteria act as tiny antennas, capturing sunlight. The energy is then conveyed to photosystems at the base of the phycobilisome. Image credits: © 2022 RIKEN SPring-8 Center
Phycobilisomes are packages of proteins and chromophores that function as antennas for catching light in photosynthesis. They are situated on the surface membranes of blue-green algae (cyanobacteria) and other algae.
Phycobilisomes absorb light at wavelengths that are difficult for other light-absorbing photosynthetic proteins, such as photosystems I and II, to utilize. Each algal species has its own unique phycobilisome structure. Gaining insights into these structures will boost our knowledge of efficient energy production from sunlight.”
Keisuke Kawakami, RIKEN SPring-8 Center
Phycobilisomes exist in five shapes, among which the cyanobacterium known as Thermosynechococcus vulcanus has the most familiar one—a half-sphere that comes with a fan-like arrangement of rods protruding from the core. Through the cores and rods, light energy is absorbed and later transmitted to photosystems I and II.
Currently, with cryo-electron microscopy, Koji Yonekura, also at the RIKEN SPring-8 Center, and his group have examined the arrangement and role of the phycobilisome of T. vulcanus, and compared it with those of four earlier reported species.
The scientists’ group identified that the core comprises five cylinders of proteins, instead of the more common three-cylinder arrangement, and they also found a new kind of cylinder. Every phycobilisome has multiple rods protruding from the core, made of stacked proteins and chromophores connected by linker proteins. T. vulcanus only has one kind of chromophore, known as phycocyanobilin, while certain algae have more than one.
Uncovering the structure of linker proteins and amino acids around each phycocyanobilin, and the interactions between them, is critical for understanding unidirectional energy transfer. We were surprised to find that amino-acid residues located around certain phycocyanobilins can manipulate the absorption wavelengths of those chromophores, enabling continuous and rapid energy transfer even under changing light conditions.”
Keisuke Kawakami, RIKEN SPring-8 Center
The researchers also discovered that these amino-acid residues differ slightly among algal species, perhaps as a consequence of the varying light conditions in various growth environments.
“Investigating the phycobilisome structures in different species will help us understand the diversity and evolution of algae,” concludes Kawakami. “Taking inspiration from phycobilisomes could inform the development of optical devices that exploit the mechanism of unidirectional energy transfer using a single chromophore.”
Source:
Journal reference:
Kawakami, K., et al. (2022) Core and rod structures of a thermophilic cyanobacterial light-harvesting phycobilisome. Nature Communications. doi.org/10.1038/s41467-022-30962-9