A dietary modification may be essential to improving colon cancer treatment, according to research from the University of Michigan Rogel Cancer Center.
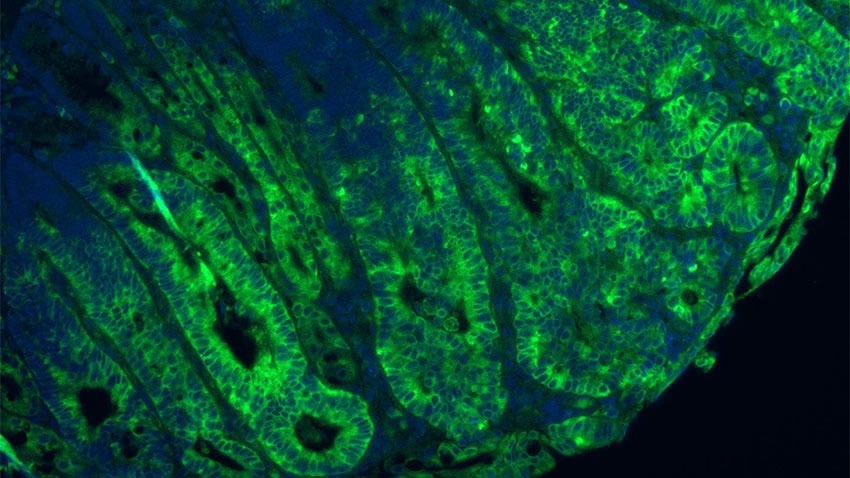 Green staining shows mTORC1 is significantly increased due to disruption in GATOR1 in a mouse model of colon cancer. Image Credit: Sumeet Solanki, PhD.
Green staining shows mTORC1 is significantly increased due to disruption in GATOR1 in a mouse model of colon cancer. Image Credit: Sumeet Solanki, PhD.
Cancer cells require nutrition in order to survive and proliferate. mTORC1 is one of the most significant nutrient sensing molecules in a cell. It is often referred to be a master regulator of cell growth since it permits cells to recognize different nutrients and so grow and multiply. When resources are scarce, cells reduce the nutrient sensing cascade and suppress mTORC1.
While mTORC1 is recognized to be hyperactive in colon cancer, the critical question is whether cancers use nutrient sensing pathways to activate the master regulator.
In colon cancer, when you decrease the nutrients available in the tumors, the cells don’t know what to do. Without the nutrients to grow, they undergo a kind of crisis, which leads to massive cell death.”
Yatrik M. Shah PhD, Study Senior Author and Horace W. Davenport Collegiate Professor, Physiology, Michigan Medicine
A low-protein diet, scientists found in cells and mice, inhibited the nutrient signaling pathway that activates a master regulator of cancer growth. The findings were reported in the journal Gastroenterology.
mTORC1 regulates how cells use nutritional signals to grow and replicate. It is known to cause cancer to become resistant to traditional treatments when it is highly active in tumors with particular mutations. A low-protein diet, particularly a reduction in two important amino acids, altered nutritional signals via a complex known as GATOR.
GATOR1 and GATOR2 combine to keep mTORC1 running. GATOR2 stimulates mTORC1 when a cell has an abundance of nutrients. When nutrients are scarce, GATOR1 inhibits mTORC1. This nutrient signaling is disrupted when particular amino acids are restricted.
Earlier attempts to suppress mTORC have concentrated on its cancer-causing signals. However, these inhibitors have serious adverse effects, and when people stop taking them, cancer reappears. According to the findings, restricting the nutrition route by restricting amino acids through a low-protein diet is an alternate method of inhibiting mTORC.
We knew that nutrients were important in mTORC regulation but we didn’t know how they directly signal to mTORC. We discovered the nutrient signaling pathway is just as important to regulate mTORC as the oncogenic signaling pathway.”
Sumeet Solanki PhD, Study First Author and Research Investigator, Rogel Cancer Center
Researchers confirmed these results in cells and mice, where they discovered that restricting amino acids inhibited cancer growth and enhanced cell death. They also examined tissue biopsies from patients with colon cancer, which revealed that high mTORC markers were associated with increased resistance to treatment and poorer outcomes.
According to Solanki, this could open up the possibility of directing treatment for people who have this marker.
Solanki added, “A low-protein diet won’t be standalone treatment. It has to be combined with something else, such as chemotherapy.”
A low-protein diet poses the danger of exacerbating muscle weakness and weight loss, which cancer patients frequently endure.
Putting cancer patients on a protein-deficient diet long-term is not ideal. But if you can find key windows—like at the start of chemotherapy or radiation—when patients could go on a low protein diet for a week or two, we could potentially increase the efficacy of those treatments.”
Yatrik M. Shah PhD, Study Senior Author and Horace W. Davenport Collegiate Professor, Physiology, Michigan Medicine
Additional research will be carried out to refine the concept of a therapeutic window to restrict amino acids. Investigators will also look into how these pathways contribute to treatment resistance and whether an inhibitor can block the GATOR complexes.
Source:
Journal reference:
Solanki, S., et al. (2022) Dysregulated amino acid sensing drives colorectal cancer growth and metabolic reprogramming leading to chemoresistance. Gastroenterology. doi.org/10.1053/j.gastro.2022.11.014.