Different types of cells, such as the heart, liver, blood, and sperm cells, have characteristics that help them perform their specific functions in the body. In general, those traits are hard-wired. A heart cell will not randomly transform into a liver cell if not stimulated.
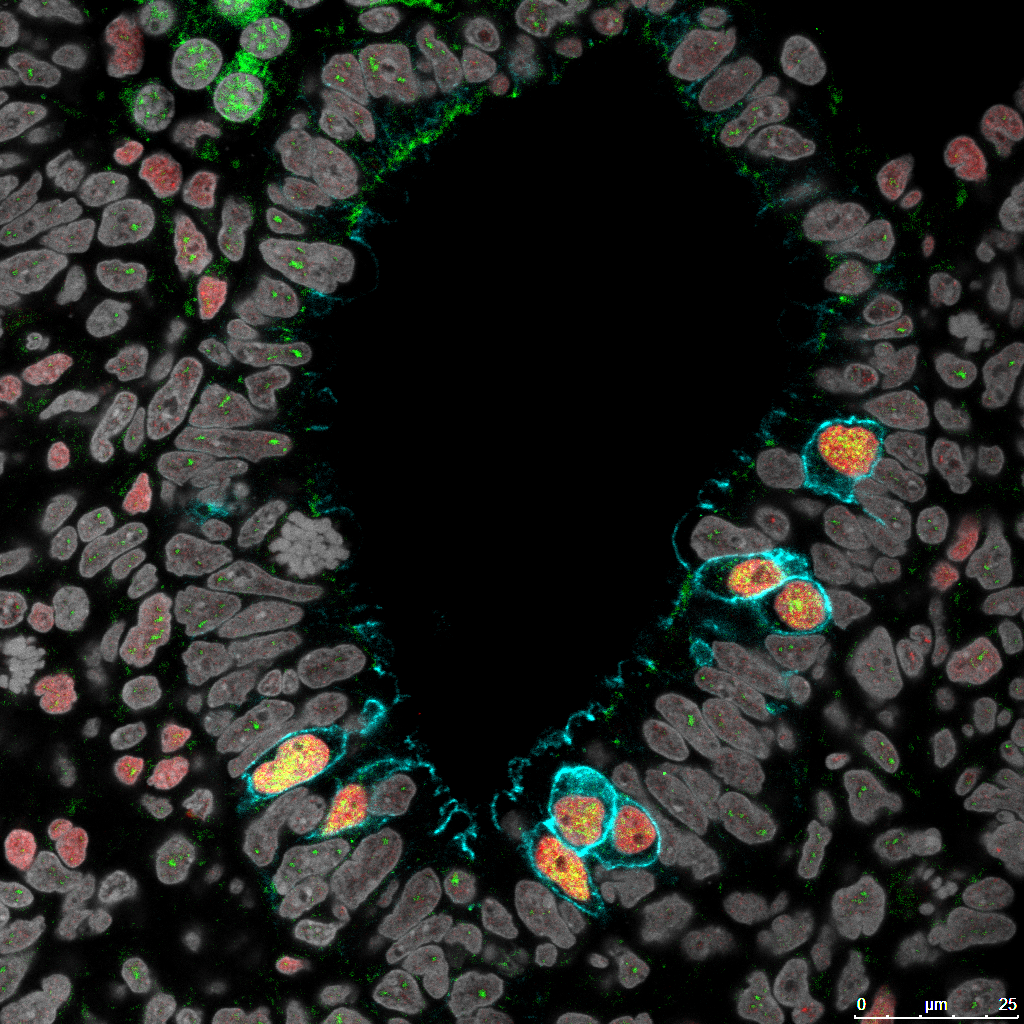 Researchers from the School of Veterinary Medicine strove to recapitulate the developmental stages of sperm biogenesis in a marmoset, a small monkey species. They began by identifying the characteristics of primordial germ cells, which give rise to sperm and eggs (labeled with fluorescent markers). Image Credit: Yasunari Seita.
Researchers from the School of Veterinary Medicine strove to recapitulate the developmental stages of sperm biogenesis in a marmoset, a small monkey species. They began by identifying the characteristics of primordial germ cells, which give rise to sperm and eggs (labeled with fluorescent markers). Image Credit: Yasunari Seita.
Scientists from the University of Pennsylvania School of Veterinary Medicine, in collaboration with contributors from the University of Texas at San Antonio and the Texas Biomedical Research Institute, have induced marmoset blood cells to procure stem cell flexibility. The stem cells were then directed to take on the characteristics of sperm precursors.
Researchers describe their step-by-step process of rewiring cells in the journal eLife. The observations, the first in the marmoset, a small monkey, open up new avenues for primate biology research and the development of novel assisted reproductive technologies such as in vitro gametogenesis, a process of producing germ cells, sperm, or eggs in a laboratory dish, similar to how in vitro fertilization produces an embryo outside the human body.
Scientists know how to generate functional sperm and egg from induced pluripotent stem cells in mice, but mouse germ cells are very different from human germ cells. By studying marmosets, whose biology more closely resembles ours, we can bridge the gap.”
Kotaro Sasaki, Assistant Professor, University of Pennsylvania School of Veterinary Medicine
The investigators first researched germ cell precursors from marmoset embryos, which had not been intensively characterized for the species before. They discovered that these early-stage cells, referred to as primordial germ cells (PGCs), carried specific molecular markers that could be followed over time.
Single-cell RNA sequencing on these cells revealed that PGCs expressed genes associated with early-stage germ cells as well as those associated with epigenetic modifications, which control gene expression.
PGCs, on the other hand, did not express genes recognized to be turned on later in the germ cell development process when precursor cells relocate to the ovaries or testes to complete their maturation.
The observations were “consistent with the notion that marmoset germ cells undergo a reprogramming process,” Sasaki says, that “turns off” specific markers, allowing PGCs to progress through the stages of germ cell development.
The patterns observed in marmoset cells strongly resemble those found in humans and other monkey species but were clearly different from those found in mice, indicating that the marmoset could be a useful model for reproductive biology research.
With that knowledge in hand, the group set about attempting to recreate the development process artificially in the lab. The first step is to convert blood cells into induced pluripotent stem cells (iPSCs), which can differentiate into a variety of other cell types.
I have a lot of experience in working with cell culture and induced pluripotent stem cells, but establishing a stable culture for the marmoset cells was a difficult part of the study.”
Yasunari Seita, Study Lead Author and Postdoctoral Researcher, University of Pennsylvania School of Veterinary Medicine
Seita developed a strategy for generating and maintaining stable cultures of iPSCs after much trial and error and applying lessons learned from mouse, human, and other studies. The addition of an inhibitor of the signaling pathway controlled by the Wnt protein, which is associated with a range of cellular functions such as cell differentiation, was important to success.
The following step was to progress from iPSCs to germ cell precursors. The protocol for this transformation was developed through extensive experimentation once again. The best method involved adding a cocktail of growth factors to effectively induce 15–40% of their culture to take on the characteristics of these germ cell precursors.
We were excited to see that efficiency and were able to expand our cultures, passaging them multiple times and seeing nice exponential growth. The cells maintained key germ cell markers but didn’t express other markers that are associated with the migration to the gonad.”
Kotaro Sasaki, Assistant Professor, University of Pennsylvania School of Veterinary Medicine
The researchers enticed these lab-grown cells to take on the qualities of later-stage germ cells in the study’s final stage. They cultured the cells with mouse testicular cells for a month using a method Sasaki and co-workers developed earlier in human cells and reported in a 2020 Nature Communications paper. As a result of the successful growth, some cells began to activate genes associated with later-stage sperm cell precursors.
Creating new methods for studying the marmoset allows the Penn and University of Texas at San Antonio teams, as well as the scientific community, to use the species as an important research model. Marmosets, for instance, have cognitive functioning that is similar to human cognitive functioning in many ways, which could lead to new insights in neuroscience.
Marmosets represent a new avenue for studying normal and abnormal development as well as fertility for Sasaki’s group, which is most interested in the development of the reproductive system.
Kotaro Sasaki concludes, “When you think about the clinical applications of an assisted reproductive technology like in vitro gametogenesis, there are a lot of ethical, legal, and safety concerns that could arise. We definitely need a good preclinical model to explore before we move to human clinical translation.”
Source:
Journal reference:
Seita, Y., et al. (2023) Efficient generation of marmoset primordial germ cell-like cells using induced pluripotent stem cells. eLife. doi.org/10.7554/eLife.82263.