In the human body, virus particles are identified by pattern recognition receptors (PRRs) either within or on the cell surface. When a receptor is activated, a signaling cascade is initiated, which results in the production and release of signaling molecules like interferons and cytokines. These messenger molecules notify neighboring immune cells of the viral infection, triggering an immune response.
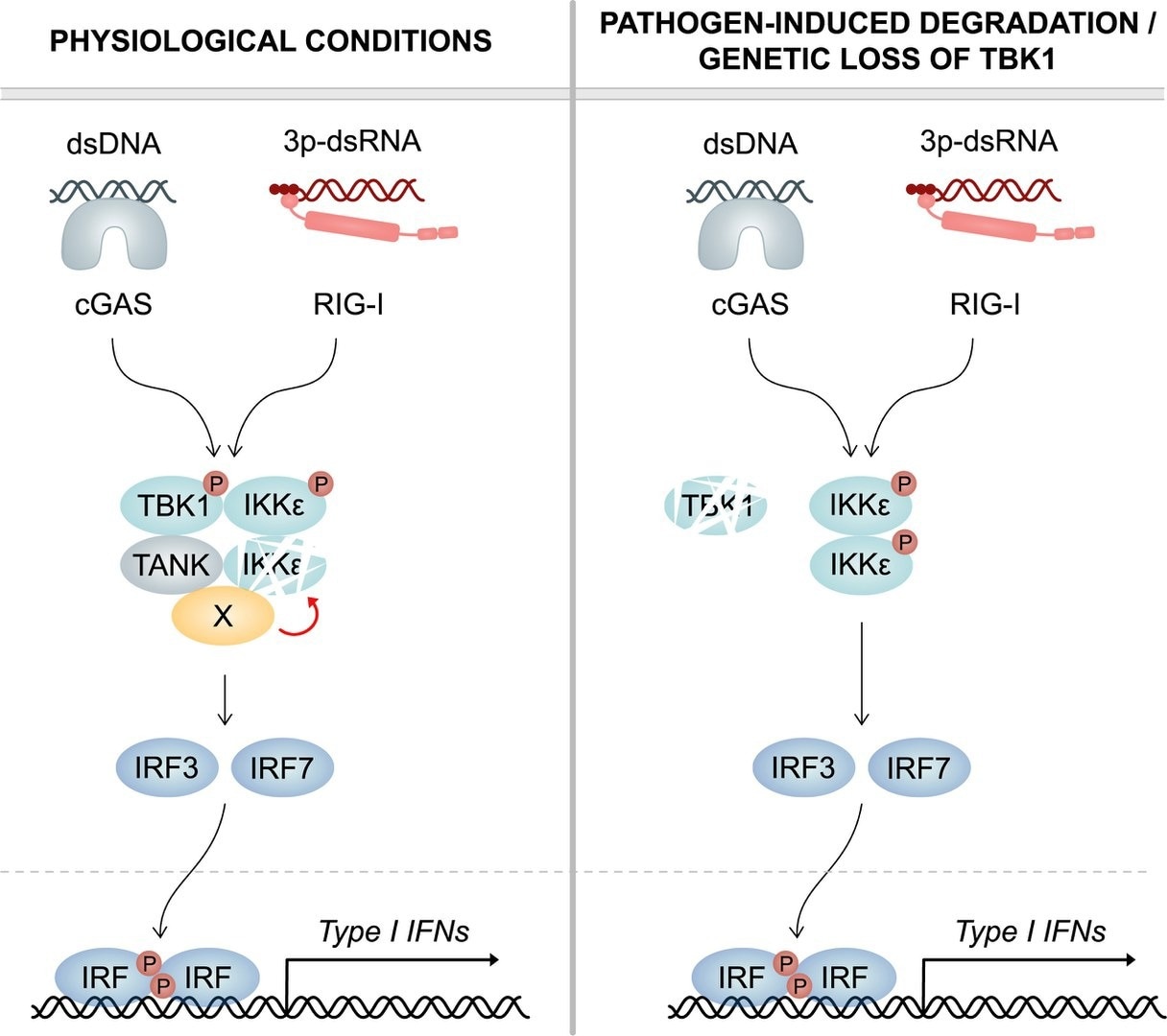 Left: Under physiological conditions—TBK1 continuously induces the degradation of its cognate kinase IKKepsilon. For this purpose, TBK1 recruits an as yet unidentified cofactor (X) via its scaffold protein TANK, which destabilizes IKKepsilon. Upon infection, both TBK1 and IKKepsilon contribute to the production of interferon (type I IFN). Right: Upon pathogen-induced degradation or genetic loss of TBK1, it no longer reduces IKKepsilon protein stability. Thus, IKKepsilon protein levels are greatly enhanced, which compensates for the deficiency of TBK1. This ensures an unrestrained type I IFN response and efficient clearance of infection. Image Credit: © Figure: Frontiers in Immunology.
Left: Under physiological conditions—TBK1 continuously induces the degradation of its cognate kinase IKKepsilon. For this purpose, TBK1 recruits an as yet unidentified cofactor (X) via its scaffold protein TANK, which destabilizes IKKepsilon. Upon infection, both TBK1 and IKKepsilon contribute to the production of interferon (type I IFN). Right: Upon pathogen-induced degradation or genetic loss of TBK1, it no longer reduces IKKepsilon protein stability. Thus, IKKepsilon protein levels are greatly enhanced, which compensates for the deficiency of TBK1. This ensures an unrestrained type I IFN response and efficient clearance of infection. Image Credit: © Figure: Frontiers in Immunology.
The TANK Binding Kinase 1 is a part of this signaling cascade (TBK1). When PRRs identify viral particles, TBK1 is activated. In turn, TBK1 stimulates two transcription factors that enter the nucleus and elicit the transcription of interferon and cytokine genes.
Susceptibility to viral infections
Point mutations in the TBK1 gene might well result in TBK1 function loss. In humans, this appears as clinical susceptibility to viral infections. Surprisingly, this effect does not occur if TBK1 is not expressed and is completely absent in the cell.
Surprisingly, a complete absence of TBK1 expression in humans is not associated with a reduced antiviral response.”
Martin Schlee, Professor, Institute of Clinical Chemistry and Clinical Pharmacology, University Hospital Bonn
It was previously unknown why a total loss of TBK1 expression is better tolerated in terms of immunocompetence than a TBK1 mutation impacting kinase function.
The Bonn scientists have now offered an explanation for these earlier unexplained observations.
“A second enzyme that is very similar to TBK1 plays an important role in this: the IkB kinase epsilon, or IKKepsilon for short,” elaborates Dr Julia Wegner, first author of the study.
IKKepsilon, like TBK1, functions downstream of PRRs and regulates interferon expression. The two proteins are also structurally very similar, with more than 60% sequence homology. A surprising discovery is that TBK1 has a direct effect on IKKepsilon.
In myeloid cells, we could show that TBK1 regulates the expression of the related kinase IKKepsilon.”
Dr Julia Wegner, Study First Author, University Hospital Bonn
No half measures
IKKepsilon's stability is reduced by TBK1. This process is unrelated to the enzymatic function of the protein.IKKepsilon’s stability is reduced by TBK1. This process is unrelated to the enzymatic function of the protein.
“Accordingly, TBK1 that is nonfunctional due to point mutation is still able to destabilize IKKepsilon, This leads to a continuous degradation of the kinase IKKepsilon in human immune cells,” narrates Prof. Gunther Hartmann, director of the Institute of Clinical Chemistry and Clinical Pharmacology and spokesperson of the ImmunoSensation2 Cluster of Excellence.
As a result, loss of TBK1 expression increases the abundance of IKKepsilon. This system guarantees that an antiviral immune response can occur even when TBK1 is not present. The loss of function of TBK1 caused by point mutations, on the other hand, does not prevent the destabilization and degradation of IKKepsilon, resulting in the inactivation of both factors for viral defense. The result is an increased susceptibility to viral infections.
Weapons of a virus
Elevated levels of IKKepsilon in a healthy organism can thus make up for the loss of TBK1. This is especially important when viruses particularly seek to destroy the body’s own defenses. Herpes simplex virus 1 (HSV-1), human immunodeficiency virus (HIV), and severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) can all cause TBK1 degradation. Furthermore, several bacterial species are capable of degrading TBK1.
Our data clearly show that human immune cells have an important backup mechanism. They are able to maintain an effective antiviral response even when pathogen-induced degradation of TBK1 occurs. Furthermore, the mechanism also takes effect in the case of genetic loss of TBK1.”
Dr Julia Wegner, Study First Author, University Hospital Bonn
Source:
Journal reference:
Wegner, J., et al. (2023). Increased IKKϵ protein stability ensures efficient type I interferon responses in conditions of TBK1 deficiency. Frontiers in Immunology. doi.org/10.3389/fimmu.2023.1073608.