Genes and their regulatory relationships form complex networks that govern cell differentiation trajectories. However, predicting and understanding cell fate decisions using bottom-up network topology is still side-lined.
 Cell growth-induced bifurcation and cell fate decision. Image Credit: Shenzhen Institute of Advanced Technology.
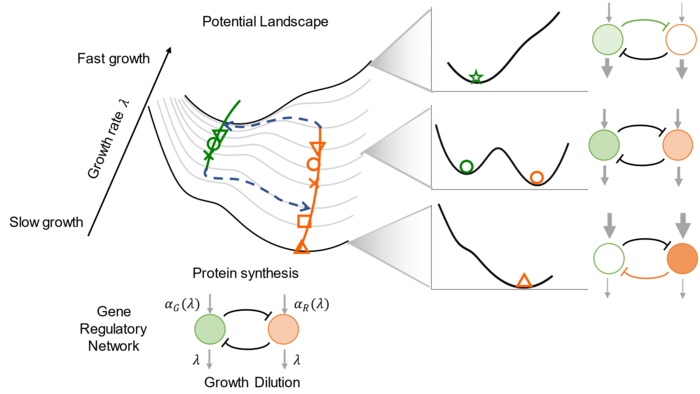
Cell growth-induced bifurcation and cell fate decision. Image Credit: Shenzhen Institute of Advanced Technology.
A study team guided by Prof. Xiongfei Fu of the Chinese Academy of Sciences’ Shenzhen Institute of Advanced Technology (SIAT) revealed recently how the global regulation factor, cellular growth rate, reshapes the cell-fate-decision landscape.
On March 23rd, 2023, the study was published in Nature Chemical Biology.
To investigate the extent to which cellular growth rate impacts phenotypic states, the investigators used a toy model of a classic synthetic genetic circuit, the toggle switch. They discovered that the gene expression response to changes in growth rate was unbalanced, resulting in a growth-rate-dependent phase transition between the toggle’s available phenotypic states.
They also investigated the toggle’s bistability under different growth conditions, discovering that the steady states bifurcate at a critical value of the cell’s growth rate. They measured gene expression capacities at various growth rates and suggested a mathematical model to explain the bifurcation caused by the unbalanced relationship between gene expression and growth rates.
To further verify the model’s capabilities, we constructed toggles with tuned intrinsic parameters and successfully predicted their steady-state properties.”
Xiongfei Fu Study Corresponding Author and Professor, Shenzhen Institute of Advanced Technology, Chinese Academy of Sciences
This global regulatory mechanism is not dependent on any specific regulatory factors. It can emerge or program a new gene expression pattern with a spatiotemporal order in any gene in the cell-fate-decision network.
“It is hard to disentangle the global factors, such as growth rate, from the cell-fate-decision processes,” added Prof. Fu hinting that the growth rates can vary as a result of the fate decision. “It's a chicken and egg decision,” Prof. Fu stated.
Investigators can, however, use quantitative synthetic biology to reconstruct orthogonal networks that insulate host genetic networks. They can then assess how growth rate impacts gene expression and promotes the bifurcation of cell phenotypic states.
Source:
Journal reference:
Zhu, J., et al. (2023). Unbalanced response to growth variations reshapes the cell fate decision landscape. Nature Chemical Biology. doi.org/10.1038/s41589-023-01302-9.