The removal of obsolete and damaged cell components by the body is crucial for fighting diseases like tuberculosis (TB), which establish themselves inside human cells, according to researchers from the Francis Crick Institute.
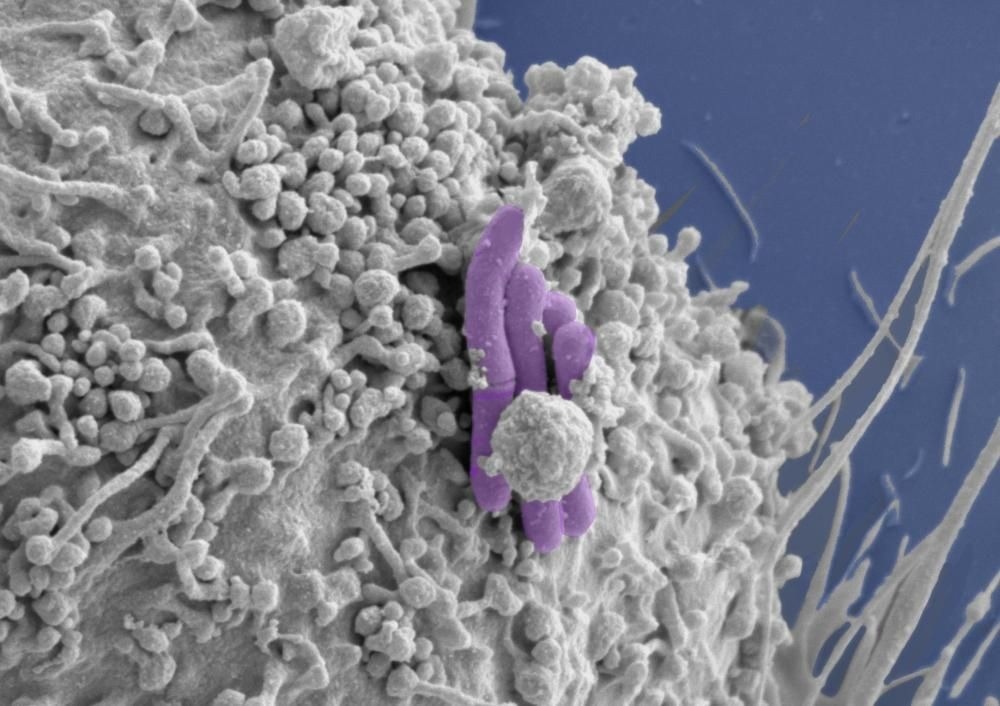 Image of a macrophage (grey) infected with M. tuberculosis (pseudocolored purple), the bacilli bacteria that cause TB. Image Credit: Tony Fearns (Host Pathogen Interactions in Tuberculosis Lab)
Image of a macrophage (grey) infected with M. tuberculosis (pseudocolored purple), the bacilli bacteria that cause TB. Image Credit: Tony Fearns (Host Pathogen Interactions in Tuberculosis Lab)
If this natural mechanism can be used in combination with new treatments, it could be possible to replace or optimize the use of antibiotics, particularly in situations where bacteria have developed drug resistance.
In their study, which was released in Nature Microbiology on 23rd March 2023 in advance of World TB Day on March 24th, 2023, the team investigated genes important for bacteria’s ability to avoid autophagy, a system that cells employ to self-destruct when stressed or infected.
From specialized stem cells known as induced pluripotent stem cells, which can develop into any type of cell in the body, they created human immune cells known as macrophages. After that, they altered the macrophages’ capacity for autophagy using methods for genome editing.
The bacterial infection took hold when genes essential for autophagy were deleted from the cells and Mycobacterium tuberculosis (the bacilli that cause TB) was introduced. As a result, the altered cells multiplied more and mass host cell death occurred.
These findings support the idea that autophagy plays a significant role in preventing intracellular infections like tuberculosis. If this route can be improved or enhanced, it may open up new possibilities for combating antibiotic resistance by increasing the potency of already available antibiotics or providing a treatment option in situations where bacteria have developed resistance.
I first studied the role of autophagy in infection during my PhD, so it is incredible to see renewed interest in this field. Using the latest technologies, we have been able to show a key role for this pathway in controlling infection.”
Max Gutierrez, Principal Group Leader, Francis Crick Institute
Gutierrez added, “As immunotherapies have harnessed the immune system to fight cancer, boosting this immune defense with a host-directed therapy, could be a valuable new tool in the fight against infections, particularly those becoming resistant to antibiotics.”
The scientists further confirmed the significance of autophagy in human defenses by validating their findings using macrophages derived from blood samples.
Antibiotic resistance is a huge threat to our health so it’s incredibly important to understand how our bodies fight infection and where there might be room for improvement.”
Beren Aylan, Study Joint First Author and PhD Student, Francis Crick Institute
Aylan stated, “TB is a great example of where targeting our own immune defenses could be really effective, because it takes a very long course of different antibiotic treatments to effectively remove the infection. Anything that can be done to more effectively remove bacteria, could also make a huge difference to the cost and accessibility of treatments.”
The group is currently preparing to search for drug compounds that could potentially be utilized to increase autophagy in a targeted way.
Gutierrez concluded, “Boosting the autophagy pathway is not as simple as it might seem. This is because all parts of the body use autophagy as a way to recycle old and damaged cells. In order to safely increase autophagy in the location of infections, we need to target the pathway in macrophages alone.”
Source:
Journal reference:
Aylan, B., et al. (2023). ATG7 and ATG14 restrict cytosolic and phagosomal Mycobacterium tuberculosis replication in human macrophages. Nature Microbiology. doi.org/10.1038/s41564-023-01335-9