Chloroplasts and mitochondria are responsible for producing energy in nature, which is essential for creating sustainable, artificial cells in the lab. In addition to being “the powerhouses of the cell,” as the adage from middle school biology goes, mitochondria are also one of the most difficult intracellular components to artificially clone.
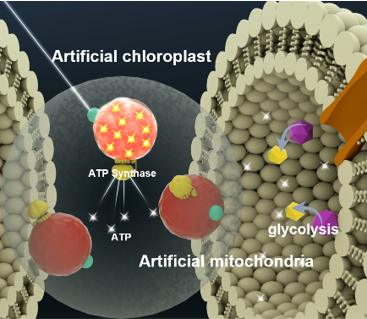 Concept of artificial chloroplasts and mitochondria within a liposome for self-sustaining energy generation through photosynthesis and cellular respiration. Image Credit: Biological Interface Group, Sogang University
Concept of artificial chloroplasts and mitochondria within a liposome for self-sustaining energy generation through photosynthesis and cellular respiration. Image Credit: Biological Interface Group, Sogang University
Researchers from Harbin Institute of Technology in China and Sogang University in South Korea recognized the most promising developments and most difficult obstacles of synthetic mitochondria and chloroplasts in Biophysics Reviews, published by AIP Publishing.
If scientists can create artificial mitochondria and chloroplasts, we could potentially develop synthetic cells that can generate energy and synthesize molecules autonomously. This would pave the way for the creation of entirely new organisms or biomaterials.”
Kwanwoo Shin, Study Author and Lab Head, Department of Chemistry, Sogang University
Chloroplasts in plants use sunlight to transform water and carbon dioxide into glucose. Both plants and animals have mitochondria, which use the breakdown of glucose to create energy.
When a cell generates energy, it frequently stores and transfers that energy using a molecule known as adenosine triphosphate (ATP). When the cell degrades ATP, the energy that drives the cell’s processes is released.
Shin added, “In other words, ATP acts as the main energy currency of the cell, and it is vital for the cell to perform most of the cellular functions.”
The team outlines the elements needed to build synthetic mitochondria and chloroplasts and notes that proteins are the most crucial components for proton transport, molecular rotary machinery, and ATP synthesis.
The components that make up the organelles that produce energy have been reproduced in various other studies. The most promising studies focus on the complicated energy-generating process’ intermediary steps. Researchers have increased energy efficiency by tying together the protein and enzyme sequence.
Enabling self-adaptation in dynamic situations to maintain a steady supply of ATP remains one of the most difficult tasks in the effort to reconstruct the energy production organelles. Before synthetic cells become self-sustainable, future research must look at ways to enhance this limiting factor.
The authors emphasize the significance of developing artificial cells with biologically plausible energy production strategies that mirror natural processes. The entire cell being replicated could generate future biomaterials and provide insights into the past.
Shin concluded, “This could be an important milestone in understanding the origin of life and the origin of cells.”
Source:
Journal reference:
Park, H., et al. (2023). Artificial organelles for sustainable chemical energy conversion and production in artificial cells: Artificial mitochondrion and chloroplasts featured. Biophysics Reviews. doi.org/10.1063/5.0131071