In Europe, Clostridioides difficile infection results in severe diarrhea and leads to the death of around 20,000 patients, every year. It is one of the most common hospital-acquired infections. The disease needs to be cured by fecal microbiota transplantation when it relapses.
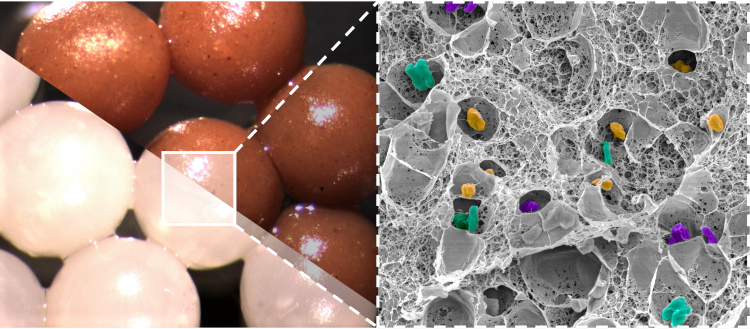 Alginate microparticles containing isolated bacterial strains (white particles) and a fecal transplant (brown particles), with a zoom on the structure of the microparticles by scanning electron microscopy. Image Credit: CC BY-NC-ND 4.0 - Adèle Rakotonirina et Nathalie Boulens
Alginate microparticles containing isolated bacterial strains (white particles) and a fecal transplant (brown particles), with a zoom on the structure of the microparticles by scanning electron microscopy. Image Credit: CC BY-NC-ND 4.0 - Adèle Rakotonirina et Nathalie Boulens
This treatment is directed through a colorectal or nasogastric tube and is very challenging. Scientists, at the University of Geneva (UNIGE), in connection with the Lausanne University Hospital (CHUV), have designed small beads that could be consumed orally, radically enhancing its administration. International Journal of Pharmaceutics published this research.
Clostridioides difficile is naturally found in 15% of the population. It is a bacteria that can turn pathogenic when the defensive ‘‘barriers’’ of human intestinal flora are enfeebled. Specifically, this is the scenario following repeated and prolonged use of antibiotics. Then, Clostridioides difficile results in severe diarrhea and can result in a critical inflammation of the colon, called pseudomembranous colitis. With over 124,000 cases every year in Europe, it is fatal in around 15% of cases and is one of the most common hospital-acquired infections.
When treated using antibiotics, the infection reverses in 1/3rd of patients. Then, intestinal microbiota transplantation is suggested. The treatment includes taking intestinal flora from the healthy donor’s feces and shifting it to the infected person’s digestive tract.
However, this effective treatment, which enables the reconstitution of intestinal microbiota, is very uncomfortable: it is given via a colorectal or nasogastric tube, or through enema. Oral capsules are available, but due to their dosage (30–40 capsules in 2 days) and their size (8.2 mm wide and 23.3 mm long), their usage is also challenging.
Revolutionary Beads
A research group, from the University of Geneva (UNIGE), in close association with the Service of infectious diseases and the Service of pharmacy from the Lausanne University Hospital (CHUV), has designed a new technology that surpasses a majority of these hindrances.
Our technique allows the micro-organisms present in the donor’s stool to be encapsulated alive in small beads of about 2 millimeters, which can be taken orally.”
Adèle Rakotonirina, Study First Author and PhD Student, School of Pharmaceutical Sciences, University of Geneva
The benefit of these beads is that they include the equivalent quantity of live bacteria as the existing prescribed capsules but in a volume lesser than 10 times. To develop them, the scientists combined feces with alginate, which are sugars or biopolymers from brown algae from the family of Phaeophyceae.
This ‘‘mix’’ of feces and sugar was then immersed, drop-by-drop, in water that comprised calcium chloride. This procedure has the impact of gelling the drops. Then, the water in the drops was extracted by freeze-drying to create small, solid beads that have the potential to carry the necessary bacteria to the intestines.
Easy to Administer
When administered, these brownish beads can be easily dispersed in a liquid or food that is pleasant to eat. They also have no taste. They could therefore greatly facilitate the administration of the treatment, particularly for children.”
Eric Allémann, Last Study Author and Full Professor, School of Pharmaceutical Sciences, University of Geneva
This technology will be required to be tested in clinical trials and provides the potential new likelihood to combat the infection of Clostridioides difficile. Coating the fecal microbiota beads to other polymers in order to characterize the optimal mixture for delivering the bacteria to the intestines will be the next step for the study group.
Source:
Journal reference:
Rakotonirina, A., et al. (2023). Dry alginate beads for fecal microbiota transplantation: From model strains to fecal samples. International Journal of Pharmaceutics. doi.org/10.1016/j.ijpharm.2023.122961.