Reviewed by Danielle Ellis, B.Sc.May 22 2023
Christian Huygens recorded his observations of the synchronization of two pendulum clocks in 1665. It takes precise quantitative control to properly regulate oscillatory dynamics, which are seen in essential biological processes, including circadian clocks, segmentation, and transcription factor responses in addition to physical systems.
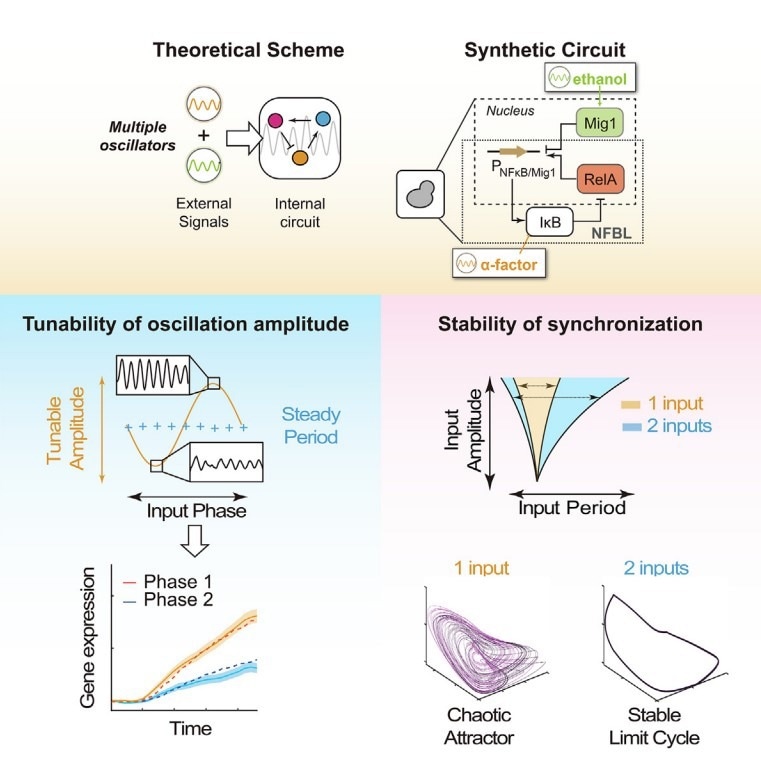 Working model outlying how three oscillations interplay and perform new dynamic behaviors. Left panel: The amplitude-only modulation of the internal oscillator enabled by tuning the relative phase of two oscillatory signals. Right panel: The stability of the entrainment is enhanced by additional external oscillations. Image Credit: Prof. Ping Wei
Working model outlying how three oscillations interplay and perform new dynamic behaviors. Left panel: The amplitude-only modulation of the internal oscillator enabled by tuning the relative phase of two oscillatory signals. Right panel: The stability of the entrainment is enhanced by additional external oscillations. Image Credit: Prof. Ping Wei
The behavior of several biological oscillators, which are impacted by various oscillatory impulses, is comprehended using Arnold Tongues’ theory.
This theory, however, oversimplifies genuine biological systems by reducing the situation to a single external signal and a single internal oscillator. Currently, the understanding of an oscillator’s behavior in the presence of two or more external oscillatory impulses is minimal.
Image Credit: ART-ur/Shutterstock.com
The Shenzhen Institute of Advanced Technology (SIAT) of the Chinese Academy of Sciences and the Niels Bohr Institute of Copenhagen University collaborated to build a synthetic oscillatory system in yeast that can respond to dual oscillatory signals to unravel this long-standing mystery.
They have demonstrated the cooperative impact of numerous oscillatory signals and the special phase control phenomena by closely combining experiment and mathematical modeling. These results pointed to a fresh method of regulating oscillatory dynamics in both biological systems that are created artificially and those that are natural.
Cell Systems published the study on May 17th, 2023.
To create a dual-responsive oscillator, the researchers tweaked a synthetic oscillator that was previously created in yeast.
The system could be synchronized by both periodic α-factor and ethanol, demonstrating that it is a tri-coupled oscillatory system, which they confirmed. Additionally, a mathematical model was created to suit the parameters and forecast additional results.
The researchers discovered that two oscillatory signals together could greatly expand the entrainment zone, raise the ratio of synchronized cells, and postpone the onset of chaos by combining modeling and experimental approaches.
These findings imply that two oscillating signals stabilized synchronization through collaboration rather than linear addition of the signals.
One of the anonymous reviewers of the study added, “I had not heard of such a surprising phenomenon before and it is quite interesting that is unveiled in a biological context. Moreover, evidence for this phenomenon is provided both theoretically and experimentally, using a synthetic circuit, which is an impressive tour de force.”
The researchers went one step further and found that the dynamics of the internal oscillator depended critically on the phase difference between two external oscillating signals. One can modify the internal oscillation amplitude while keeping the oscillation frequency constant by adjusting this phase difference.
The ideal phase difference is closely connected to the natural phase differences of the various system components under free-oscillating conditions, according to the researchers’ additional findings.
The van de pol oscillator, the p53 oscillator, and the natural NF-B oscillator were three other oscillatory systems that the authors modeled to confirm the universality of their findings. The fundamental findings were proved to be reliable and consistent across the three systems studied.
The researchers examined if adjusting the phase difference of oscillatory signals could influence downstream gene transcription as a last-ditch attempt to validate the phase regulatory mechanism.
Despite the presence of noise, they discovered that phase modulation does, in fact, considerably alter the amount of gene expression, and this is further connected to the various amplitudes of the internal oscillator.
Our novel synthetic cell signaling system, together with mathematical modeling, serve as a powerful platform to study such complex biological problems. The results presented here broadened our general understanding of biological coupled oscillators and emphasized the importance of a correct time pattern in biological regulation. We hope that these findings may inspire scientist from the much more broad disciplines.”
Ping Wei, Study Corresponding Author and Professor, Shenzhen Institute of Advanced Technology, Chinese Academy of Sciences
Source:
Journal reference:
Heltberg, M. S., et al. (2023). Coupled oscillator cooperativity as a control mechanism in chronobiology. Cell Systems. doi.org/10.1016/j.cels.2023.04.001