Salmonella enterica, a disease-causing bacterium, employs a variety of strategies to evade the human body’s defense mechanisms, much like thieves. In a new study, scientists at IISc’s Department of Microbiology and Cell Biology (MCB) identify two methods the bacterium employs to protect itself, both of which are driven by the same protein.
 Stealthy Salmonella escapes host’s defenses using two-pronged approach. Image Credit: Ritika Chatterjee.
Stealthy Salmonella escapes host’s defenses using two-pronged approach. Image Credit: Ritika Chatterjee.
When Salmonella enters the human body, each bacterial cell is enclosed in a bubble-like structure called a Salmonella-containing vacuole (SCV). When humans get infected with bacteria, the immune cells produce reactive oxygen species (ROS) and reactive nitrogen species (RNS), as well as pathways that are activated to break down these SCVs and fuse them with cellular structures called lysosomes or autophagosomes, which eliminate the bacteria.
These bacteria, on the other hand, have developed sophisticated methods to maintain vacuolar integrity, which is vital to their survival. When a bacterial cell divides, the vacuole around it divides as well, allowing each new bacterial cell to be ensconced in a vacuole. This also ensures that there are more vacuoles present than lysosomes capable of digesting them.
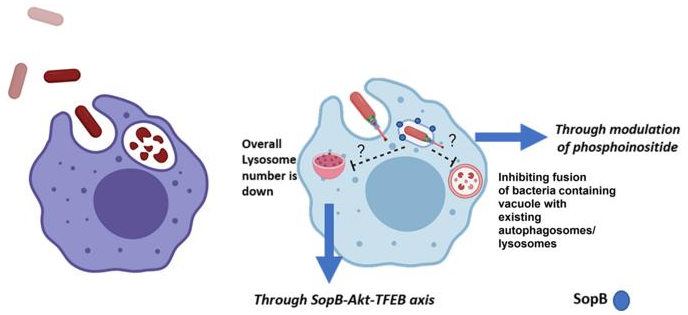
The IISc team discovered that a key protein produced by Salmonella, known as SopB, blocks the fusion of SCV with lysosomes and the formation of lysosomes, in a two-pronged attempt to protect the bacterium. The study was published in the journal Microbes and Infection.
[This] gives the upper hand to bacteria to survive inside macrophages or other host cells.”
Ritika Chatterjee, Study First Author and Former PhD Student, Department of Microbiology and Cell Biology, Indian Institute of Science
The experiments were performed on immune cell lines and immune cells isolated from mouse models.
SopB is a phosphatase, meaning it helps remove phosphate groups from phosphoinositide, a form of membrane lipid. SopB assists Salmonella in changing the dynamics of the vacuole, specifically the kind of inositol phosphates in the vacuole membrane, preventing vacuole fusion with lysosomes.
A recent study from the same team found that when host cells are infected with Salmonella, the number of lysosomes produced by the cells reduces. The researchers also discovered that mutant bacteria that were unable to produce SopB were unable to lower the number of host lysosomes. As a result, they decided to use advanced imaging techniques to investigate the involvement of SopB in the development of lysosomes.
What researchers discovered was that SopB inhibits the translocation of a critical molecule called Transcription Factor EB (TFEB) from the cytoplasm of the host cell into the nucleus. This translocation is critical because TFEB regulates lysosome production.
This is the first time we deciphered that SopB can work in a dual manner—it changes the phosphoinositide dynamics of SCV and affects TFEB’s translocation into the nucleus. While other groups have already reported the function of SopB in mediating invasion in epithelial cells, the novelty of our study lies in identification of the function of SopB in inhibiting the vacuolar fusion with existing autophagosomes/lysosomes, and the second mechanism, which provides Salmonella with a survival advantage by increasing the ratio of SCV to lysosomes.”
Dipshikha Chakravortty, Study Corresponding Author and Professor, Department of Microbiology and Cell Biology, Indian Institute of Science
Scientists believe utilizing small molecule inhibitors of SopB or TFEB activators can help prevent Salmonella infection.
In future investigations, the researchers hope to look into the effect of another host protein called Syntaxin-17, whose levels decrease after Salmonella infection.
How do the SCVs reduce the levels of Syntaxin-17? Do they exchange it with some other molecules, or do the bacteria degrade it? We [plan to] look into it next.”
Dipshikha Chakravortty, Study Corresponding Author and Professor, Department of Microbiology and Cell Biology, Indian Institute of Science
Source:
Journal reference:
Chatterjee, R., et al. (2023). Deceiving The Big Eaters: Salmonella Typhimurium SopB subverts host cell Xenophagy in macrophages via dual mechanisms. Microbes and Infection. doi.org/10.1016/j.micinf.2023.105128.