Reviewed by Danielle Ellis, B.Sc.Jun 7 2023
The world’s quickest camera capable of detecting fluorescence from single molecules has been created by scientists from Kyoto University, Okinawa Institute of Science and Technology Graduate University (OIST), and Photron Limited in Japan.
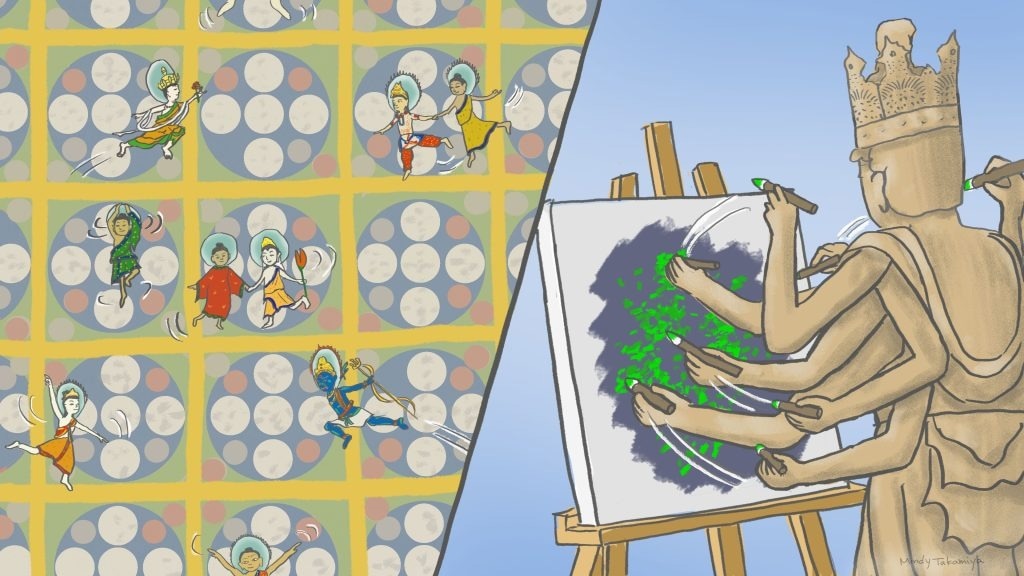 (Left) The ultrafast camera revealed molecules in the cell membrane (represented by various buddhas) appearing to dance within spaces bound by actin filaments (represented by the mandalas) and occasionally jumping over the boundaries. (Right) Pairing the ultrafast camera with SFMI allows the creation of pointillism-like images 60 times faster than previous methods. Image Credit: Mindy Takamiya/Kyoto University iCeMS
(Left) The ultrafast camera revealed molecules in the cell membrane (represented by various buddhas) appearing to dance within spaces bound by actin filaments (represented by the mandalas) and occasionally jumping over the boundaries. (Right) Pairing the ultrafast camera with SFMI allows the creation of pointillism-like images 60 times faster than previous methods. Image Credit: Mindy Takamiya/Kyoto University iCeMS
In two publications that were published in the same issue of the Journal of Cell Biology, they describe the technology and provide instances of its effectiveness.
Our work with this camera will help scientists understand how cancer spreads and help develop new drugs for treating cancer.”
Takahiro Fujiwara, Study Lead and Professor, Institute for Integrated Cell-Material Sciences
By attaching a fluorescent reporter tag to molecules of interest in a cell, single fluorescent-molecule imaging (SFMI) can show where these molecules are as well as how they are moving and interacting with one another. The team’s ultra-fast camera makes it possible for SFMI to attain its best resolution in time ever.
It is capable of detecting molecular motions that happen 1,000 times more quickly than normal video frames. It can specifically identify a molecule with a fluorescent tag affixed every 33 microseconds with a 34 nm accuracy in location or every 100 microseconds with a 20 nm precision.
Fujiwara added, “We can now observe how individual molecules dance within living cells, as if we are watching a ballet performance in a theatre.”
He emphasized that prior SFMI methodologies required the audience to infer the story from such few observations, which was equivalent to seeing the ballet once every 30 seconds. It was really challenging, and the estimates were sometimes wholly incorrect.
Furthermore, the team’s ultrafast camera vastly increased the time resolution of a prior super spatial resolution approach, which received the Nobel Prize in Chemistry in 2014.
The locations of individual molecules are recorded as little dots of around 20 nm in this older approach, generating visuals similar to the pointillism paintings of the new impressionists, pioneered by Georges Seurat.
The challenge with pointillism under the microscope has been that the image production is exceedingly slow, taking more than 10 minutes to create a single image, therefore the specimens have to be chemically preserved dead cells.
The image can be created in 10 seconds with the new ultrafast camera, which is around 60 times quicker, allowing observation of live cells.
The researchers further demonstrated the camera’s capabilities by investigating the positioning and mobility of a cancer-related receptor protein and a cellular structure known as focal adhesion.
Focal adhesion is a protein complex that binds bundles of structural proteins inside cells to the extracellular matrix. It can play a crucial role in cellular mechanical interactions with its environment, allowing cancer cells to migrate and spread.
In one investigation we found that a cancer-promoting receptor that binds to signaling molecules is confined within a specific cellular compartment for a longer time when it is activated. In another, we revealed ultrafine structures and molecular movements within the focal adhesion that are involved in cancer cell activities.”
Akihiro Kusumi, Study Corresponding Authors, Professor Emeritus, Kyoto University
Kusumi is also a professor at Okinawa Institute of Science and Technology Graduate University. The findings enabled the researchers to develop a more precise model of focal adhesion structure and function.
Many research groups throughout the world are interested in creating drugs that can block the function of focal adhesions in cancer.
These efforts will be aided by the ultrafast camera that the team created in partnership with Mr. Takeuchi of Photron Limited, a Japanese camera maker. It will reveal a greater knowledge of how these structures move and interact with other structures both within and outside of cells.
Source:
Journal references:
- Fujiwara, T. K., et al. (2023). Development of ultrafast camera-based single fluorescent-molecule imaging for cell biology. Journal of Cell Biology. doi.org/10.1083/jcb.202110160
- Fujiwara, T. K., et al. (2023). Ultrafast single-molecule imaging reveals focal adhesion nano-architecture and molecular dynamics. Journal of Cell Biology. doi.org/10.1083/jcb.202110162