Reviewed by Danielle Ellis, B.Sc.Jun 13 2023
Nuclear factor erythroid 2-related factor 2 (NRF2) is a master transcription factor with a role in maintaining oxidative stress response. It can be triggered by both redox-dependent and redox-independent pathways. The regulatory mechanisms of the redox-dependent pathway are well understood; however, the redox-independent pathway is not.
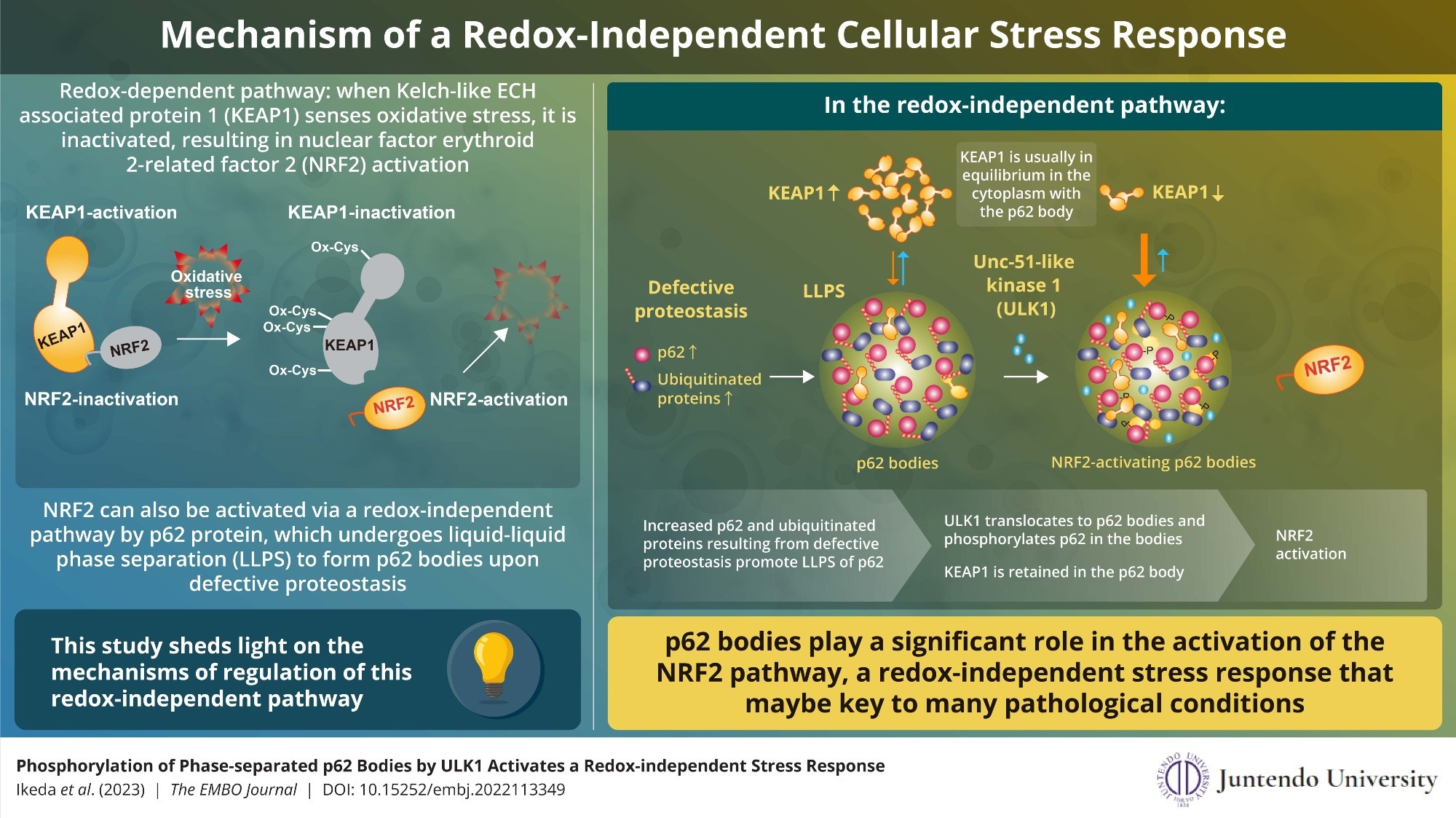 ULK1 interacts directly with and phosphorylates the biomolecular condensate, p62 body. Phosphorylated p62 retains KEAP1 within itself and activates NRF2. The findings shed new light on the redox-independent activation of NRF2, which has an important role in stress response. Image Credits: Masaaki Komatsu from Juntendo University School of Medicine
ULK1 interacts directly with and phosphorylates the biomolecular condensate, p62 body. Phosphorylated p62 retains KEAP1 within itself and activates NRF2. The findings shed new light on the redox-independent activation of NRF2, which has an important role in stress response. Image Credits: Masaaki Komatsu from Juntendo University School of Medicine
Juntendo University researchers have now discovered the roles of phase-separated liquid droplets, known as p62 bodies, in redox-independent NRF2 activation, as well as the importance of phosphorylation in this process.
Cellular stress, also known as oxidative stress, occurs when reactive oxygen species (ROS) accumulate, disrupting cellular mechanisms and potentially damaging proteins, lipids, and DNA.
All cells have robust systems in place to remove ROS and decrease oxidative stress due to their destructive nature. The nuclear factor erythroid 2-related factor 2 (NRF2)-mediated stress response is one such mechanism.
NRF2 is a master transcription factor that contributes to the reduction of oxidative stress.
NRF2 activation by redox and its subsequent involvement in stress response is well known. Kelch-like ECH-associated protein 1 (KEAP1) recognizes oxidative stress in the cell by oxidation of its particular cysteine residues in this pathway.
This oxidation alters the structure of KEAP1, causing it to lose its capacity to inhibit NRF2. As a result, NRF2 is stabilized, and a series of genes producing anti-oxidative proteins are activated, reducing and eliminating oxidative stress produced by ROS.
NRF2 can be triggered in a redox-independent manner as well. When the p62 protein links to ubiquitinated proteins due to defective proteostasis, it experiences liquid-liquid phase separation to create p62 bodies. However, the specific method of NRF2 regulation via p62 bodies was previously unknown.
Scientists in Japan have discovered how p62 bodies regulate redox-independent NRF2 activity. Professor Masaaki Komatsu and Associate Professor Yoshinobu Ichimura from Juntendo University School of Medicine, as well as Dr Nobuo N. Noda from Hokkaido University, carried out the study.
We have reported in a previous study that phosphorylation of p62 inhibits the binding of KEAP1 to NRF2 competitively, thereby disabling the NRF2-repressive function of KEAP1. However, the regulatory mechanism and the physiological functions in vivo remain largely unclear. This is important as the accumulation of phosphorylated p62 has been found to cause many intractable diseases”
Masaaki Komatsu, Professor, Juntendo University School of Medicine
The study was published in The EMBO Journal.
Utilizing sophisticated methods such as high-speed atomic force microscopy, fluorescence recovery after photobleaching, and fluorescence loss in photobleaching, the group carried out experiments in vitro and in vivo to extensively profile protein-protein interactions, cellular localization of specific components, and the impacts of LLPS-induced p62 phosphorylation during redox-independent NRF2 act.
We found that ULK1, a protein kinase, translocates to p62 bodies and then phosphorylates p62 within the bodies. The resulting phosphorylated p62 bodies retain KEAP1 within them, which drives the activation of NRF2.”
Yoshinobu Ichimura, Associate Professor, Juntendo University School of Medicine
KEAP1 normally cycles in and out of p62 bodies; however, phosphorylated p62 binds tightly to KEAP1, causing it to be maintained and sequestered within the p62 body. This results in the activation of even more NRF2. Autophagy degrades p62 bodies, which may contribute to the pathway’s inactivation. The current work broadens the antioxidative stress response and sheds fresh light on the involvement of phase separation in the process.
The significance of this redox-independent NRF2 activation was investigated using phosphomimetic p62 knock-in mouse models, in which NRF2 hyperactivation caused hyperkeratosis—a growth defect in which the outer layer of the stomach and esophageal lining thickens, resulting in stunted growth due to malnutrition.
Prof. Komatsu believes his team has provided the groundwork for future research into the mechanism and regulation of redox-independent stress responses.
Whether redox-dependent or independent, NRF2 activation is an important biological defense system. Understanding its regulatory mechanisms is crucial as its persistent activation leads to inordinate defense responses, like excessive keratinization. Our study is the first scientific validation of the physiological significance for redox-independent NRF2 activation.”
Masaaki Komatsu, Professor, Juntendo University School of Medicine
“In the case of redox-independent stress responses, activation of NRF2 is very likely regulated by phosphorylation, dephosphorylation, and autophagic degradation of p62 bodies. p62 bodies have been found to accumulate in affected cells in patients with liver disorders, neurodegenerative diseases, and cancers. Thus, research such as ours will be useful in elucidating the pathogenesis of and developing improved therapies for p62-, NRF2-, and autophagy-related diseases,” concludes Professor Komatsu.
Source:
Journal reference:
Ikeda, R., et al. (2023). Phosphorylation of phase-separated p62 bodies by ULK1 activates a redox-independent stress response. The EMBO Journal. doi.org/10.15252/embj.2022113349.