Reviewed by Danielle Ellis, B.Sc.Jun 14 2023
The immune system protects the body from intruders, like bacteria, viruses, or tumors, with its intricate network of proteins, cells, and organs. Cytotoxic T cells are specialized immune cells that can grow into short-lived effector cells that destroy infected or malignant cells in human bodies.
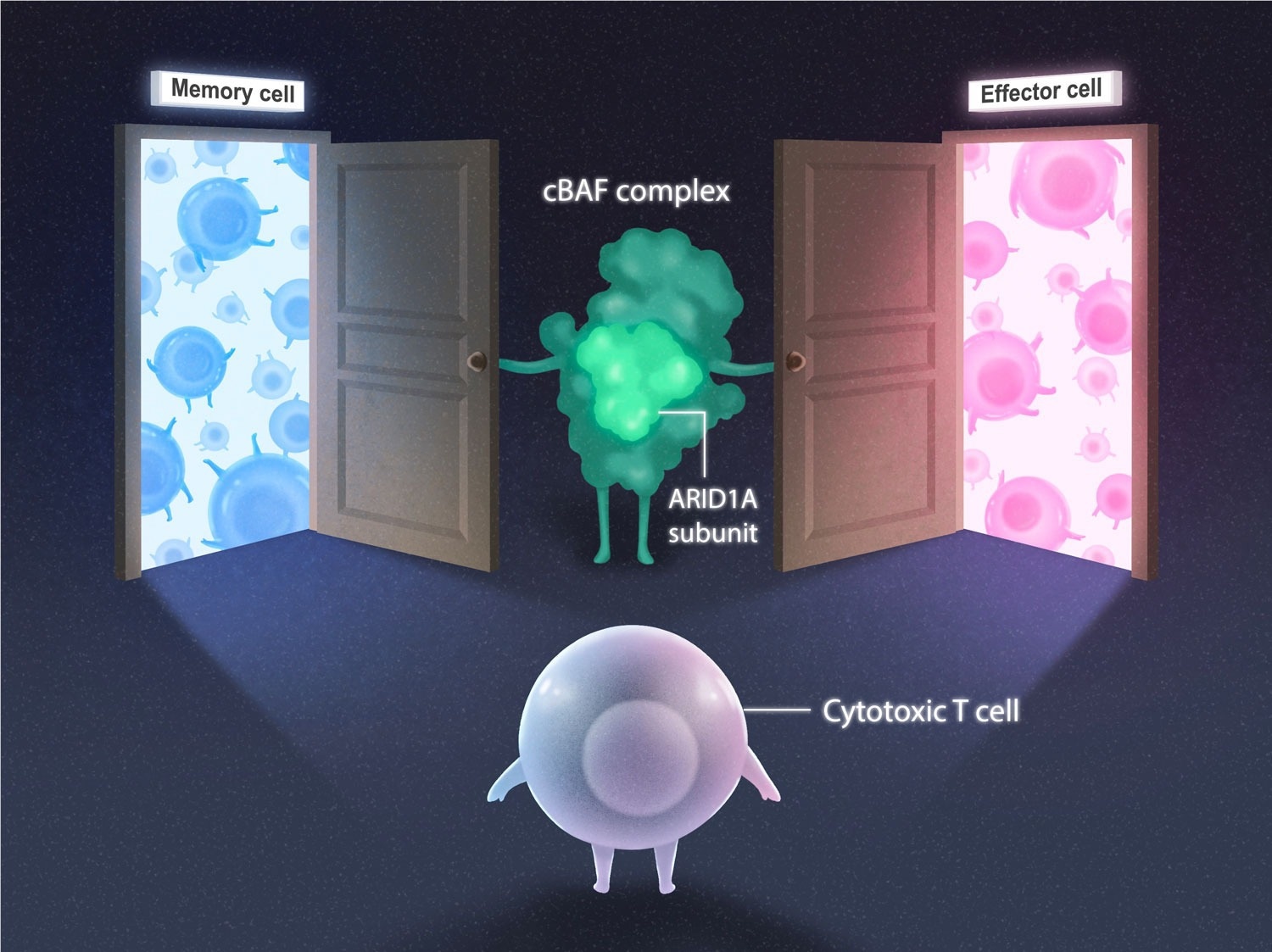 Cytotoxic T cell (purple) deciding whether to become a memory cell (blue) or effector cell (pink) subtype. The cBAF complex (green) and ARID1A subunit open the doors for both cell fate options. Image Credit: Salk Institute.
Cytotoxic T cell (purple) deciding whether to become a memory cell (blue) or effector cell (pink) subtype. The cBAF complex (green) and ARID1A subunit open the doors for both cell fate options. Image Credit: Salk Institute.
A small proportion of those effector cells survive an infection and develop into memory cells, which “remember” infections and respond when they reemerge. However, little was known about what causes cytotoxic T cells to differentiate into effector and memory T cell subtypes.
Professor Susan Kaech and Associate Professor Diana Hargreaves of the Salk Institute have revealed that a protein complex named cBAF can open or close genetic “doors” to influence T cell fate. The findings, published in the journal Immunity on June 13th, 2023, shed light on how T cells fight and remember infections, paving the path for the development of more effective vaccinations and cancer therapies.
We’ve shown how T cells transform into specialized subtypes during an infection and what happens when they mess up the genetic blueprint. Without this genetic blueprint, T cells may not be able to resolve or remember infections, or could possibly become cancerous, like we see in T cell lymphoma. Finding that the cBAF protein complex is essential in creating different T cell subtypes opens up new questions, like can we control this process to optimize immune cell function for cancer therapeutics?”
Susan Kaech, Professor, Senior and Co-Corresponding Author and Director, NOMIS Center for Immunobiology and Microbial Pathogenesis, Salk Institute
Susan Kaech is also the holder of the NOMIS Chair.
The method by which cytotoxic T cells evolve into effector and memory T cells can be explained by examining how they are created—a process that is dependent on genetic instructions encoded in chromatin.
Chromatin is made up of a combination of genetic code (DNA) that functions as instructions and proteins and RNA that control and safeguard those instructions. Rearranging chromatin within a cell exposes or conceals certain parts of the genetic code, hence changing the available instructions.
When instructions are available, proteins known as transcription factors determine whether and how frequently they are read.
The scientists were aware that chromatin functions similarly to scaffolding, sheltering the genetic code within and protecting it from transcription factors. To access those instructions, transcription factors are likely to require the assistance of chromatin-remodeling cBAF, which can signal proteins and RNA to disclose genetic code for transcription factors.
Chromatin remodeling is essential in determining the identities of cells. In other cell types, the chromatin remodeling complex cBAF was known to play a major role in cellular differentiation. We wanted to know if cBAF was important for cytotoxic T cell differentiation, too.”
Bryan McDonald, Study Co-First Author and Graduate Student, Salk Institute
ARID1A is one of many subunits that comprise the cBAF complex. To investigate the role of ARID1A in chromatin remodeling in T cells, the researchers studied T cells from mice with and without ARID1A subunits. The researchers investigated for changes in chromatin structure, protein location, and genetic code accessibility after a week-long viral infection (to stimulate the T cell immune response).
The chromatin landscape evolved immensely over the course of the infection. cBAF complexes bearing the ARID1A subunit were crucial in rearranging chromatin so transcription factors could access the genetic code that instructs the development of different T cell subtypes with specialized functions.”
Brent Chick, Study Co-First Author and Graduate Student, Salk Institute
Cytotoxic T cells could not become the short-lived, rapid-response effector subtype without ARID1A. The absence of ARID1A did not prevent cytotoxic T cells from transforming into their longer-lived memory kind, but memory cells produced in the absence of ARID1A were less effective at reacting to viral reinfection.
Finally, ARID1A was required for the development of memory T cells in organs such as the lungs and small intestine. Tissue-resident memory T cells cannot grow without ARID1A.
As the team expected, ARID1A was the pivotal player. And the process was now clear: during infections, cBAF complexes with ARID1A open various chromatin “doors” to reveal genetic code to transcription factors, which then control the fate of cytotoxic T cells.
The discovery of this unique method opens the door to further research into how chromatin remodeling, genetic coding, and transcription factors interact to contribute to a successful immune system response.
“Our research demonstrates that removing ARID1A from the immune response equation may greatly damage our ability to fight infection. I hope that our findings inspire new questions in infectious disease and cancer research—and that we have demonstrated the value of asking those questions collaboratively,” states Hargreaves, co-corresponding author and holder of the Richard Heyman and Anne Daigle Endowed Developmental Chair
The scientists intend to investigate this method further using human T cells. They also want to know how this process affects memory T cells, which are important prognostic factors for human tumors.
Source:
Journal reference:
McDonald, B., et al. (2023). Canonical BAF complex activity shapes the enhancer landscape that licenses CD8+ T cell effector and memory fates. Immunity. doi.org/10.1016/j.immuni.2023.05.005.