Reviewed by Danielle Ellis, B.Sc.Jun 28 2023
Researchers from the Max Delbrück Center for Molecular Medicine in the Helmholtz Association in Berlin and the Centre for Genomic Regulation (CRG) in Barcelona have discovered how cells speed changes to their identity known as “cell fate conversion.”
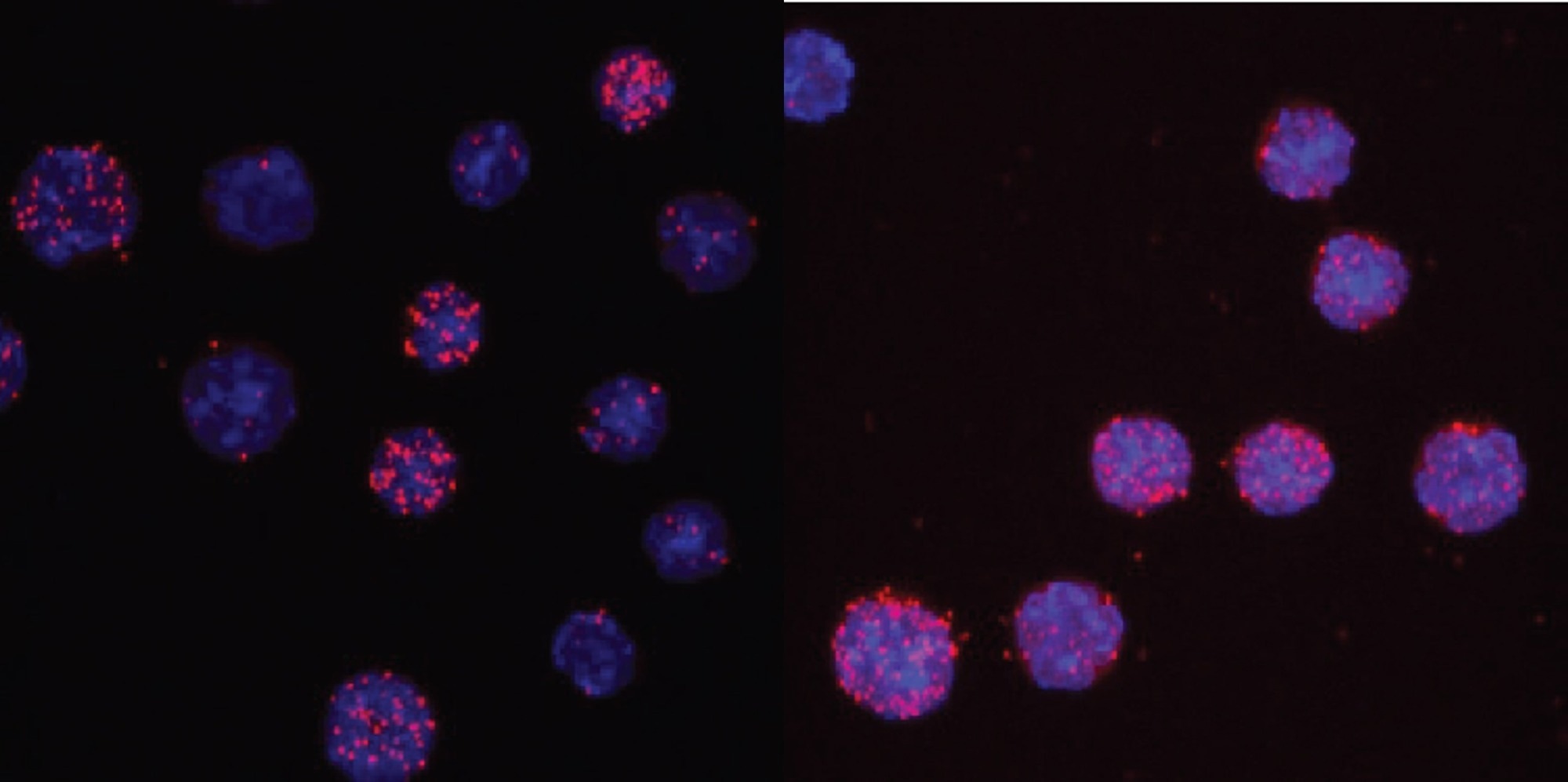 C/EBPαR35A interacts more strongly with PU.1 than unmated C/EBPα, shown here by an assay that scores the proximity of antibodies against the two proteins. Image Credit: Thomas Graf/Centre for Genomic Regulation
C/EBPαR35A interacts more strongly with PU.1 than unmated C/EBPα, shown here by an assay that scores the proximity of antibodies against the two proteins. Image Credit: Thomas Graf/Centre for Genomic Regulation
The study, which was just published in the journal eLife, has significance for cancer research since mistakes in cell fate determination frequently lead to cancer. The research could ultimately result in novel techniques for speeding or controlling the molecular processes involved in the development of cancer.
C/EBPα (CCAAT/enhancer-binding protein alpha), a protein that directs the transformation of B lymphocytes into macrophages, another type of immune cell, is crucial to the study. A protein called C/EBPα is a transcription factor, which affects the rate of transcription, the initial step that results in the activation or silencing of protein production.
Transcription factors bind to specific DNA sequences in the regulatory regions of genes. Transcription factors are essential for cell growth and function, as well as the differentiation of a particular type of cell into another during development.
Enzymes can change C/EBPα, like many other proteins, for example, by adding a methyl group to particular amino acids. The interactions of the protein can be significantly impacted by these alterations.
The scientists discovered that leaving one particular arginine residue of C/EBPα unmethylated significantly speeds up the process by which B cells transform into macrophages.
The study also discovered that the enzyme Carm1 is involved in the methylation of this specific arginine residue. Carm1-deficient mice are resistant to induced forms of acute myeloid leukemia, according to a previous study.
The researchers hypothesize that the processes discovered in this study could help explain why the unmethylated form of C/EBPα is a stronger inducer of macrophage differentiation than its methylated counterpart. Since macrophages are non-dividing cells, this could limit cancer cell development.
By understanding how cell fate conversion can be accelerated or directed, we uncover new clues for cancer research. For example, targeting the balance between methylated and unmethylated forms of C/EBPα can help us understand how immune cells differentiate and eventually lead to new ideas for treating certain forms of leukemia.”
Dr Thomas Graf, Study Senior Author and Senior Scientist, Centre for Genomic Regulation
The discovery was made while utilizing human and mouse models to look for C/EBP mutations that impact the efficacy of cell fate conversion.
When the authors investigated a mutant form of C/EBPα termed C/EBPαR35A, they discovered the location of the crucial amino acid within C/EBPα. This mutation significantly increased the pace at which B cells could be converted into macrophages.
C/EBPα induces cell conversion by interacting with another transcription factor termed PU.1, which is required for immune cell development and is already present in B cells.
C/EBPαR35A showed a substantially higher contact affinity with PU.1, accelerating the rate at which the two proteins combined to silence B cell-associated genes and activate macrophage-associated genes.
An example of an epigenetic mechanism is C/EBPα’s methylation. These are systems that alter how the genome, the handbook found inside each and every cell of the human body, is read.
Drugs that affect epigenetic mechanisms as described in the present study may indeed alter the function of transcription factors and correct cells that went astray, such as seen in cancer and leukemia.”
Dr Achim Leutz, Study Senior Author and Professor, Max-Delbrück-Center
Dr Leutz further added, “In this novel mechanism PU.1 is triggered by C/EBPα to switch from a B cell regulator into a macrophage regulator, an elegant ‘on-off’ mechanism that ensures the faithful formation of a mature cell type, avoiding the formation of ‘confused’ cells often seen in blood cancers. Therefore, drugs might be found that target this mechanism to correct such defects.”
The study's authors assert that the two processes are interconnected and that there is still much to learn about what influences the speed and directionality of cell fate decisions. How, for instance, do the many cell types in the body develop sequentially from stem cells?
Applications for a better understanding of how cells become who they are and how to control the process range from regenerative medicine to increasing the effectiveness of cancer treatments.
Source:
Journal reference:
Garcia, G. T., et al. (2023). Carm1-arginine methylation of the transcription factor C/EBPα regulates transdifferentiation velocity. eLife. doi.org/10.7554/eLife.83951