iPS cells have had a significant impact on biology and medicine, and they are expected to increase regenerative medicine. Clinical trials with various cell types derived from iPS cells have been performed since 2014, when a sheet of retinal pigment epithelial cells obtained from iPS cells was transplanted into patients with age-related macular degeneration.
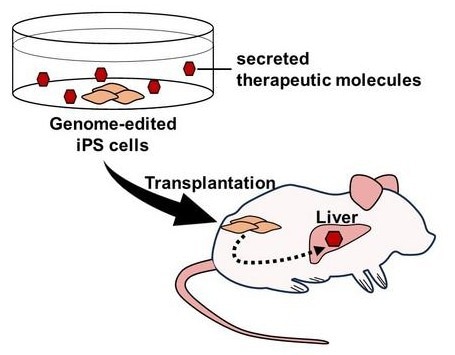 Genome editing enables the expression and secretion of therapeutic molecules from induced pluripotent stem (iPS) cells. Furthermore, transplantation of these cells allows for the delivery of therapeutic molecules to organs and tissues in vivo. Image Credit: Tokyo Metropolitan Institute of Medical Science.
Genome editing enables the expression and secretion of therapeutic molecules from induced pluripotent stem (iPS) cells. Furthermore, transplantation of these cells allows for the delivery of therapeutic molecules to organs and tissues in vivo. Image Credit: Tokyo Metropolitan Institute of Medical Science.
While iPS cells obtained from healthy individuals have been used thus far, it is anticipated that in the future, transplantation therapy using iPS cells will be improved through genetic modification.
As a proof of concept, researchers used a Fabry disease mouse model to address this possibility. The genetic deficiency of α-Galactosidase A (GLA) causes Fabry disease, which results in the accumulation of its substrates like globotriaosylceramide (Gb3) and globotriaosylsphingosine (Lyso-Gb3).
Researchers previously created an engineered enzyme, modified α-N-acetylgalactosaminidase (mNAGA), to treat Fabry disease by changing the substrate specificity of NAGA, a GLA paralog, into that of GLA. Because mNAGA retains NAGA’s original antigenicity, it has no immunological cross-reactivity with GLA while possessing GLA enzymatic activity. They investigated whether transplanting iPS cells secreting mNAGA via genome editing could supply GLA activity in vivo.
Researchers used TALEN-mediated knock-in to the AAVS1 site, a safe harbor locus, to produce iPS cells that secrete mNAGA. Furthermore, to rule out any immunogenic reactions induced by the endogenous GLA of iPS cells in patients, researchers disrupted the GLA gene using CRISPR-Cas9.
When Fabry model cardiomyocytes and fibroblasts with no GLA activity were co-cultured with mNAGA-secreting iPS cells, mNAGA-expressing cells restored GLA activity in vitro.
The iPS cells secreting mNAGA were then transplanted into the testes of Fabry disease model mice. GLA activity in the liver was improved significantly after 7 or 8 weeks, but there was no recovery in the heart, kidney, or blood plasma. They also measured the levels of Gb3 and Lyso-Gb3 in the liver, but there was no noticeable decrease in the substrates.
Because of the small amount of mNAGA secreted by the transplanted iPS cells, GLA activity in the liver was insufficient to reduce Gb3 or Lyso-Gb3. However, in the future, genome editing may make it possible to increase the amount of mNAGA secreted.
There is also the possibility of delivering mNAGA directly to organs and tissues that require GLA activity. Transplantation of a cardiomyocyte sheet derived from iPS cells secreting mNAGA, for instance, directly delivers mNAGA to the heart. Furthermore, while this research focused on Fabry disease, the same strategy can be used to treat other diseases.
Using genome-edited iPS cells that secrete therapeutic molecules, the current research revealed the effectiveness of cell therapy. These genome-edited iPS cells may be used not only for cell transplantation but also as a drug delivery system.
Source:
Journal reference:
Nakajima, I., et al. (2023). In Vivo Delivery of Therapeutic Molecules by Transplantation of Genome-Edited Induced Pluripotent Stem Cells. Cell Transplantation. doi.org/10.1177/09636897231173734.