Reviewed by Danielle Ellis, B.Sc.Jul 19 2023
How do brain cells, or neurons, distinguish their own processes and those of other neurons when they send out processes to connect with other neurons? A molecule known as clustered protocadherin (Pcdh) plays an important role in this puzzle.
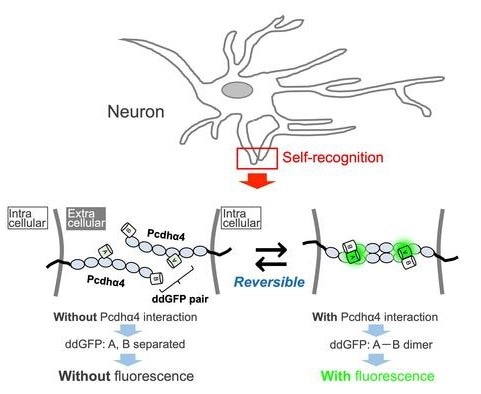 Detection of self-recognition by IPAD. Image Credit: Takashi Kanadome
Detection of self-recognition by IPAD. Image Credit: Takashi Kanadome
Investigators from SANKEN (The Institute of Scientific and Industrial Research) and Osaka University’s Graduate School of Frontier Biosciences described the development of a sensor to look at Pcdh interactions in live neurons, inching a step closer to solving this mystery. The study was published in the journal iScience.
Millions of neurons in the brain form trillions of connections with one another. To accomplish this, each neuron sends out tiny processes that develop and travel until they find the processes of another cell with which to connect.
However, since each cell has so many processes going on, cells can make connections with themselves rather than with others by accident. Pcdh, which is expressed in different combinations on the surface of each neuron, is one way to avoid this.
Image Credit: Giovanni Cancemi/Shutterstock.com
Pcdh plays a role in cell adhesion; if two neuronal processes have the same combination of Pcdh molecules, the molecules bind to each other. If the combinations are even subtly different, they are regarded as “other” rather than “self”’ and do not bind.
Even though there are traditional techniques for identifying molecular interactions between cell surfaces, they only demonstrate when the molecules bind and then dissociate. Osaka University researchers wanted to address this issue.
We developed a fluorescent-based sensor that we named IPAD, or Indicators for Protocadherin Alpha 4 interactions upon Dimerization. This sensor allows us to see not only interactions between processes but also the dissociation of these interactions for the first time.”
Takashi Kanadome, Study Lead Author and Researcher, Osaka University
This new method does have some drawbacks. Its fluorescence, for instance, is much duller than that witnessed with older techniques, and it is unable to distinguish connections between processes from the same cell and those from two different cells with the same combinations of Pcdh on the surface.
Despite its current drawbacks, we think that our new sensor will be useful for a number of different research applications. The development of IPAD is an important step toward a better understanding of the neuronal recognition of self/other.”
Takashi Kanadome, Study Lead Author and Researcher, Osaka University
The sensors have a wide range of potential applications. The technique could be used to create a variety of fluorescent sensors to visualize neuronal self-connectivity, which has been linked to brain disorders such as autism and epilepsy. Enhanced treatments for these disorders may result from a deeper understanding of neuronal self-connectivity.
Source:
Journal reference:
Kanadome, T., et al. (2023) Visualization of trans-interactions of a protocadherin-α between processes originating from single neurons. iScience. doi:10.1016/j.isci.2023.107238.