Reviewed by Danielle Ellis, B.Sc.Jul 25 2023
Even though infections can be presented with several different symptoms, one common symptom is known to be the loss of fat and muscle, a process known as wasting.
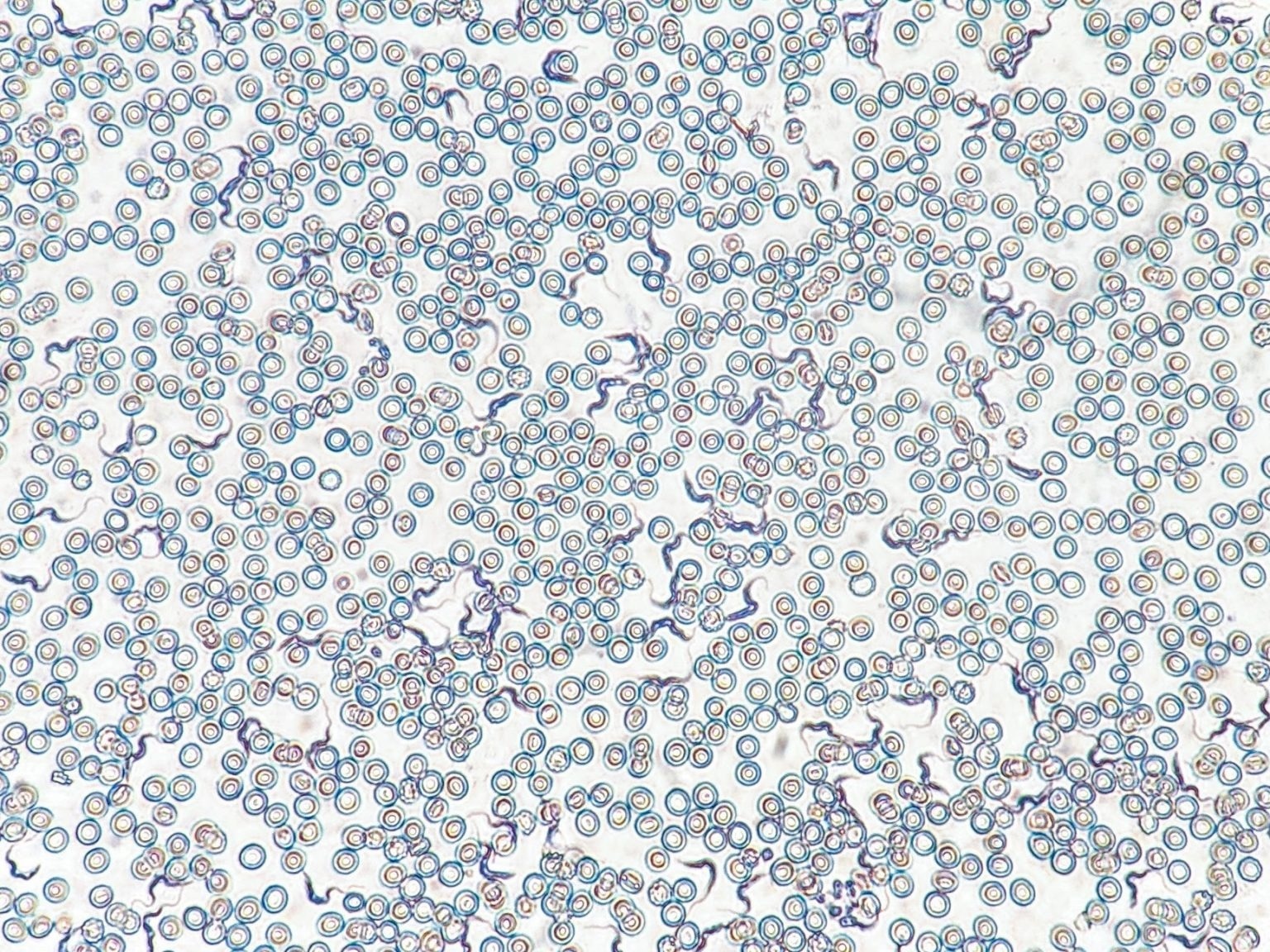 Even though infections can be presented with several different symptoms, one common symptom is known to be the loss of fat and muscle, a process known as wasting.
Even though infections can be presented with several different symptoms, one common symptom is known to be the loss of fat and muscle, a process known as wasting.
Researchers from Salk wished to know if wasting helped fight infections.
Scientists in Professor Janelle Ayres’ lab found out the wasting response to T. brucei infection in mice happens in two phases, each controlled by various immune cells. While fat loss did not be of use in the fight against infection, muscle loss did—a shocking clue that some wasting might help control illness.
The study performed could inform the development of highly effective therapeutics that spare people from wasting and increase the knowledge of how wasting causes an impact on survival and morbidity throughout cancers, chronic illnesses, infections, and more.
The study outcomes have been reported in the journal Cell Reports on July 24th, 2023
We often make assumptions that conditions like wasting are bad, since they often coincide with higher mortality rates. But if instead we ask, what is the purpose of wasting? We can find surprising and insightful answers that can help us understand the human response to infection and how we can optimize that response.”
Janelle Ayres, Study Senior Author, Legacy Chair and Head, Molecular and Systems Physiology Laboratory, Salk Institute
A huge amount of energy is required to defend the body from an invader. Earlier studies performed had this immune-related energy consumption and also the unlucky impact of wasting. However, Ayres and the team were curious to know if wasting could be advantageous and not just a side effect.
Specialized immune cells known as T cells respond slowly to infections, but when they do respond, they adapt to fight the specific infection. Ayres was interested to know if it was these T cells that are responsible for wasting. If T cells are accountable for the condition, that would denote wasting is not just an unproductive side effect of energy-hungry immune cells.
The cells of interest are known as CD4+ and CD8+ T cells. CD4+ T cells result in the fight against infection and could also encourage the activity of CD8+ T cells, which has the potential to kill invaders and cancerous cells. Frequently, the two T cell types work collectively, so the scientists hypothesized their role in wasting might be a cooperative effort, too.
To figure out the relationship between CD4+ and CD8+ T cells and wasting, the scientists turned to the parasite T. brucei. Since T. brucei lives in fat and could block the adaptive immune response—which consists of T cells—it was an ideal model infection for their questions concerning fat wasting and how T cells intercede in that process.
The team analyzed 1) the role of CD4+ and CD8+ T cells during T. brucei infection and 2) how eliminating CD4+ and CD8+ T cells altered the mortality rates, longevity, parasite symptoms, and amount of parasites present in infected mice.
The scientists discovered that CD4+ T cells acted first and initiated the process of fat wasting. Later, but entirely independently of the fat wasting, CD8+ T cells started the process of muscle wasting.
The CD4+ T cell-induced fat wasting showed no effect on the ability of the mice to fight T. brucei or to survive infection. But, the CD8+ T cell-induced muscle wasting, in opposition to the conventional assumptions about wasting, assisted the mice combat T. brucei and survive the infection.
Our discoveries were so surprising that there were times I wondered if we did something wrong.”
Samuel Redford, Study First Author and Current Visiting Researcher, Salk Institute
Redford added, “We had striking results that mice with fully functioning immune systems and mice without CD4+ T cells lived the same amount of time—meaning, those CD4+ T cells and the fat wasting they caused were completely disposable in fighting the parasite. And beyond that, we found that normally cooperative T cell subtypes were working totally independently of one another.”
The study outcomes demonstrate the significant role of immune cells in both fat and muscle wasting and the necessity to comprehend the function of such responses for therapeutic interventions to be informed.
We can learn so much about our immune systems by looking at the environments and infections we have co-evolved with. While T. brucei is an interesting and important case, what is exciting is extrapolating our findings to understand, treat, and overcome any disease that involves immune-mediated wasting—parasites, tumors, chronic illnesses, and so much more.”
Janelle Ayres, Study Senior Author, Legacy Chair and Head, Molecular and Systems Physiology Laboratory, Salk Institute
In the future, the research group will analyze the T cell mechanism in other mammals and ultimately humans. Also, they want to examine in an elaborate way why muscle wasting is taking place and why CD4+ and CD8+ T cells play such separate roles.
Source:
Journal reference:
Redford, S. E., et al. (2023) CD4+ T cells regulate sickness-induced anorexia and fat wasting during chronic parasitic infection. Cell Reports. doi.org/10.1016/j.celrep.2023.112814.