Reviewed by Danielle Ellis, B.Sc.Aug 28 2023
Researchers at Cincinnati Children’s Hospital reveal how damage to the cell’s energy factory causes muscle wasting. Closing a pore in the mitochondrial membrane in gene-edited mice prevents disease progression.
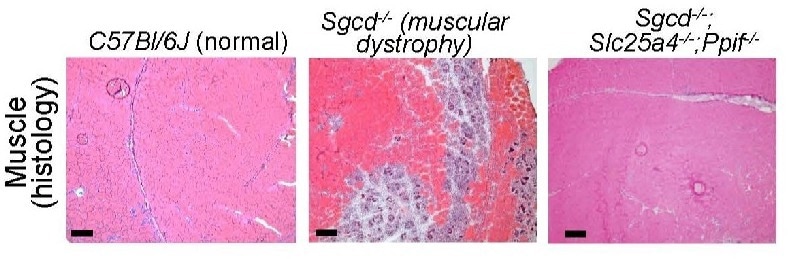
Image Credit: Cincinnati Children’s Hospital Medical Center
Since the Jerry Lewis telethons started in the 1960s, millions of people have become acquainted with muscular dystrophy (MD), an otherwise rare disease.
Over the years, the medical community has gained a lot of knowledge, including the fact that there are more than 30 closely related disorders that can cause the gradual muscle degeneration that deprives a child's ability to walk and finally disrupts other organ functions. An estimated 250,000 people in the United States have muscular dystrophy. While enhanced treatments are allowing many people to live longer lives, no cure has been discovered.
Cincinnati Children's recent research reports a completely new approach to preventing the muscle-wasting symptoms of MD. The study focuses on the role of mitochondria, a tiny organelle within our cells that converts nutrients into the energy cells require to survive.

Image Credit: metamorworks/Shutterstock.com
The research was published in Science Advances on August 25th, 2023.
We have isolated the primary disease-causing component of muscular dystrophy to the mitochondrial permeability pore. If we prevent this pore from functioning, dystrophic disease in the mouse models we studied almost completely vanishes. We see the protection lasting past one year of life in the mouse, which translates to about 40 years of life for a human.”
Jeffery Molkentin PhD, Study Corresponding Author, Cincinnati Children’s Hospital Medical Center
Molkentin is a well-known expert in the fundamental science of muscle cell function and formation. He is the Co-Executive Director of Cincinnati Children’s Heart Institute and Division of Cardiovascular Biology. He has been researching muscular dystrophies for over two decades.
Molkentin notes that this discovery was made by observing the outcomes of mice that had been genetically altered to lack two genes that control the formation of mitochondrial permeability transition pore (MPTP). Beyond this early success, much more research will be required to create a safe and effective treatment for individuals with MDs.
Mitochondria Take Center Stage
Mitochondrial organelles have their own membrane. When subjected to oxidative stress or a pathologic calcium ion (Ca2+) overload, mitochondria open a pore in their protective membrane. The influx of surplus calcium induces the organelle to burst, resulting in the death of muscle fibers and, eventually, the wasting of entire muscle groups.
This mitochondrial pore-regulated cell death method has been noted in other conditions, such as heart muscle damage following heart attacks and neurodegenerative diseases such as Alzheimer’s disease, Huntington’s disease, and amyotrophic lateral sclerosis (ALS).
Molkentin and coworkers disclose the underlying mechanisms when this mitochondria-destroying process occurs in MDs in this study. The researchers discovered that two genes in mice, Slc25a4 and Ppif, collaborate to facilitate unwanted pore formation. Controlling either one alone decelerated MD progression, but the absence of both together stalled it.
We found direct evidence that these genes produce required components that govern cell death, which opens a previously unrecognized pathway for potentially treating MDs and other necrotic diseases.”
Jeffery Molkentin PhD, Study Corresponding Author, Cincinnati Children’s Hospital Medical Center
Challenges Ahead
Molkentin believes that developing a medication to safeguard mitochondria in muscle cells will necessitate much more research.
CypD is a protein produced by the mouse gene Ppif. Researchers discovered that the immune-suppressing drug cyclosporin A can hinder this protein years ago, but long-term use of the drug at high doses carries a high risk of side effects. In animal models of MD, the drug only moderately slows the disease.
Meanwhile, the mouse gene Slc25a4 codes for the protein ANT1. This protein is not the target of any medications. Because the compounds identified to attach with this protein are toxic, new compounds would be required.
If a nontoxic ANT inhibitor can be identified, that also can target the mitochondrial pore, our results suggest that combined treatment with low dosages of a CypD inhibitor could be a novel therapeutic strategy. Such a treatment could provide benefits independently or in combination with other gene therapies.”
Jeffery Molkentin PhD, Study Corresponding Author, Cincinnati Children’s Hospital Medical Center
It is too early to say if such a treatment would “cure” muscular dystrophy. This study concentrated solely on skeletal muscle. More research would be required to determine whether the mitochondria-protecting strategy would also safeguard against MD-related heart damage or other organ dysfunctions.
Source:
Journal reference:
Bround, M. J., et al. (2023). ANT-dependent MPTP underlies necrotic myofiber death in muscular dystrophy. Science Advances. doi.org/10.1126/sciadv.adi2767.