Reviewed by Danielle Ellis, B.Sc.Aug 28 2023
The non-specific lethal (NSL) complex is a chromatin-associated factor that has been shown in both fruit flies and mammals to regulate the expression of thousands of genes. Abrogation of the NSL genes causes the organism to die, which gives rise to the complex’s unusual name.
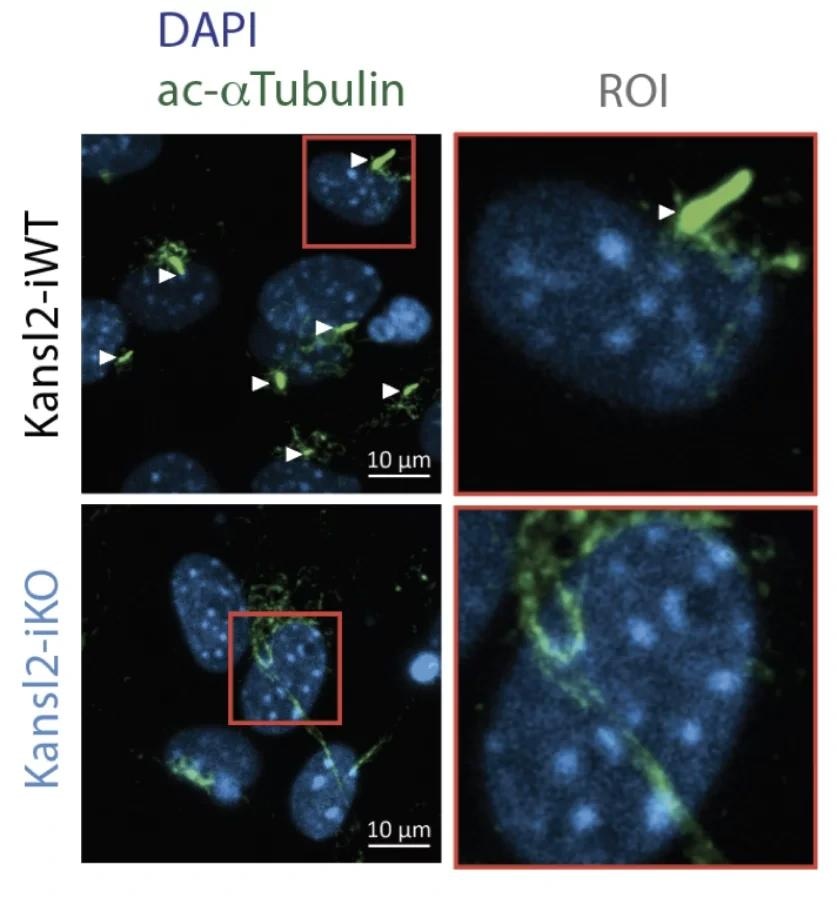 Cilia are absent in KANSL2-deleted cells (bottom). Microscopic images of mouse embryonic fibroblasts. The cilia are stained with ac-α-tubulin (green) and indicated by a white arrowhead. Nuclei are stained with DAPI (blue). Image Credit: © MPI of Immunobiology and Epigenetics, Akhtar.
Cilia are absent in KANSL2-deleted cells (bottom). Microscopic images of mouse embryonic fibroblasts. The cilia are stained with ac-α-tubulin (green) and indicated by a white arrowhead. Nuclei are stained with DAPI (blue). Image Credit: © MPI of Immunobiology and Epigenetics, Akhtar.
The NSL complex has now been identified as a “master” epigenetic regulator of intraciliary transport genes across various cell types and species by Max Planck researchers. The study found that the NSL complex “turned on” this class of genes regardless of whether a cell has cilia or not.
Furthermore, the researchers discovered that this class of cilia-associated genes is critical for the function of kidney podocytes, a highly specialized cell type that, paradoxically, lacks cilia. These discoveries have far-reaching ramifications for ciliopathies and kidney disease.

Image Credit: nobeastsofierce/Shutterstock.com
Cilia are thin, eyelash-like extensions of cells’ surfaces. They have a wide range of functions, such as mechanosensors and chemosensors, and play an important role in many signaling pathways. The organelle has encountered a remarkable but sinister career transformation in the last few decades.
It progressed from an organelle whose function was unknown to becoming a key player in the pathogenesis of a wide range of diseases. These ciliopathies cause a variety of symptoms, including hearing loss, visual impairment, obesity, kidney disease, and mental disability. Different gene mutations hinder cilia formation, maintenance, and function, arising in these ciliopathies, which can be multi-organ, syndromic disorders in some cases.
Cilia assembly, upkeep, and function are all dependent on a process known as “intraciliary transport.” To ensure a constant supply of materials, intraciliary transport system components “walk” on the microtubule to deliver cargo between the cell body and the ciliary tip.
Mutations in genes encoding intraciliary transport machinery components could result in ciliopathies. Asifa Akhtar’s lab recently identified the NSL complex as a transcriptional regulator of genes known for their roles in the intraciliary transport system of cilia across multiple cell types in a study published in the journal Science Advances.
The NSL Complex Enables Intraciliary Transport
In fruit flies, mice, and humans, the NSL complex is a powerful epigenetic modifier that controls thousands of genes. However, the majority of the NSL complex’s functions are still unknown and have only recently begun to be unraveled.
Previous research from our lab indicates that the NSL complex controls many pathways critical for organismal development and cellular homeostasis.”
Asifa Akhtar, Director, Immunobiology and Epigenetics, Max-Planck-Gesellschaft
The complex is a histone acetyltransferase (HAT) complex that facilitates the genes for activation and consists of several proteins.
Think of gene regulation as a team effort with different players. One important player is the NSL complex. It puts special marks on the histone proteins on which the DNA is wrapped around in the nucleus, like putting up green flags. These flags tell other regulators to switch on specific genes. We now found that the NSL complex does exactly this for a group of genes linked to moving materials within cilia.”
Tsz Hong Tsang, Study First Author, Max-Planck-Gesellschaft
Without Components of the NSL Complex, the Cell Cannot Build a Cilium
The intraciliary transport system is necessary for the formation of a functional cilium. The intraciliary transport system is used by the cell to move material from the cilium base to the growing tip, similar to how a tower is built. The investigators used mouse cells in the research to examine the functional consequences of the NSL complex’s loss in the cells.
They discovered that fibroblast cells devoid of the NSL complex protein KANSL2 could neither activate nor assemble cilia.
As cilia are the sensory and signaling hubs for cells, loss of KANSL2 leads to the inability of cells to activate the sonic hedgehog signaling pathway, which plays important roles in the regulation of embryonic development, cell differentiation, and maintenance of adult tissues as well as cancer.”
Asifa Akhtar, Director, Immunobiology and Epigenetics, Max-Planck-Gesellschaft
These sensory organelles, despite their small size, are extremely important to cells. Ciliopathies, which impact organs as vast and varied as the kidney, liver, eye, ear, and central nervous system, continue to pose difficulties in biological and clinical research.
The investigators at the Max Planck Institute in Freiburg hope that their study of the NSL complex’s role has offered valuable insights into the regulation of these organelles and the genes associated with them, thereby benefiting human health.
Consequences of NSL Loss in Non-Ciliated Cells
Cilia can be found in almost every cell type in the human body. This describes why ciliopathies can impact so many distinct organs and tissues, but there are also non-ciliated cells. Mature glomerular podocytes, which are special filtration cells in the kidney, are one of the cell types that lack cilia.
“Interestingly, we found that podocytes also express these intraciliary transport genes that are regulated by the NSL complex. So, we wondered what would happen if they are unable to switch on these genes,” notes Tsz Hong Tsang.
The scientists found that the loss of KANSL2 causes changes in microtubule dynamics in non-ciliated mouse podocytes. Microtubules are cytoskeletal components that are responsible for cell mechanical stability and intracellular transport of different organelles.
While mature podocytes lack cilia, they do have specialized cell processes that extend from the cell body called primary and secondary processes, the functions of which heavily rely on cytoskeletal components.
Even though the defect in ciliated cells appears to be milder, the Akhtar lab discovered that cytoskeletal defects are most probably the cause of extreme glomerulopathy and kidney failure witnessed in mice lacking the NSL complex. These and other extraciliary functions of intraciliary transport genes may help explain the complexities of ciliopathies’ symptoms.
Source:
Journal reference:
Tsang, T. H., et al. (2023). Transcriptional regulation by the NSL complex enables diversification of IFT functions in ciliated versus nonciliated cells. Science Advances. doi.org/10.1126/sciadv.adh5598.