Reviewed by Danielle Ellis, B.Sc.Oct 3 2023
Fatty liver disease is on the rise worldwide, affecting up to 30% of people in Western countries. In addition to drinking, obesity and diabetes are major risk factors.
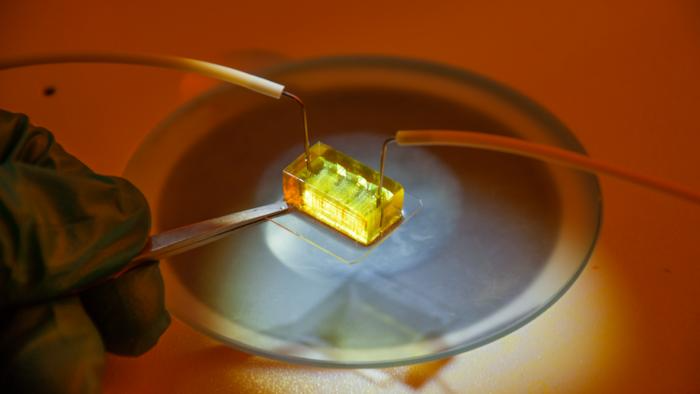 3D tissue culture platform, enabled by massively parallel arrays of high-resolution 3D printed microperfusion hydrogel channels that functionally mimic the human liver’s blood vessels. The platform supports a long-term culture of liver models with dimensions of several millimeters at physiologically relevant cell densities, which is difficult to achieve with other methods. Photo: DTU Health Tech. Image Credit: DTU Health Tech.
3D tissue culture platform, enabled by massively parallel arrays of high-resolution 3D printed microperfusion hydrogel channels that functionally mimic the human liver’s blood vessels. The platform supports a long-term culture of liver models with dimensions of several millimeters at physiologically relevant cell densities, which is difficult to achieve with other methods. Photo: DTU Health Tech. Image Credit: DTU Health Tech.
There is a huge focus on seeing if there is anything you can do to inhibit this explosion of fatty liver disease medically.”
Niels Bent Larsen, Professor, Department of Health Technology, Technical University of Denmark
His team at DTU Health Tech, along with colleagues from Norway, Sweden, Germany, and the Czech Republic, has created a novel method that could speed up the process of discovering new drugs to treat liver diseases. Their findings were recently published in the journal Acta Biomaterialia.
Larsen added, “To find new drugs efficiently, you need models, and you want them to mimic the real thing - its structure and function - as closely as possible. So, what we have done in our labs is to make a real mini liver. Basically, we 3D print a bundle of artificial blood vessels and pour live liver cells in between them.”
Image Credit: Explode/Shutterstock.com
“Because the sides of these artificial blood vessel are very close together, we can supply the liver cells with the same conditions they have inside our liver. By sending in liquid with nourishment and oxygen through the artificial blood vessels we can keep them running for months as a fused liver-like tissue with retained liver functions,” Larsen added.
Model Recreates Real Environment
The human liver is made up of microscopic units called acini, each of which has diverse functions and requires varied quantities of oxygen and nutrition. The zone nearest to the blood supply will receive the most oxygen, which is important for investigating metabolic disorders since it supports oxidative energy metabolism and glucose production.
The oxygen in the blood going through the acinus is used by the liver cells, thus the zone farthest from the blood supply receives significantly less oxygen. This is the site of enzymatic mechanisms that convert poisons and drugs from the small intestine into safer compounds but it is also the site of toxic byproducts that can kill liver cells and result in irreversible liver damage.
Current approaches for modeling the liver are prone to rapidly losing characteristics. They also lack zones with varying oxygen levels and functions, which are required to evaluate how the liver realistically reacts to various drugs.
The researchers examined how the common painkiller paracetamol impacted cells at various oxygen levels and discovered that cells in the low-oxygen zone were more responsive to the drugs. Since paracetamol is exclusively digested in this zone, it is a good sign that the liver model is viable. They also examined the proteins produced by the cells and discovered that their distribution between zones was similar to that of human liver acini.
Larsen concluded, “To my knowledge, our model is the only one out there that can recreate this real environment only from having the liver cells themselves produce the natural gradual change in oxygen and metabolites through the acinus. I believe it shows great promise that we have been able to make real, living tissue that we can monitor and experiment on in 3D and in an environment that closely mimics the real liver. This gives us a huge amount of control and will enable drug developers to speed up their testing significantly.”
Source:
Journal reference:
Wesseler, M. F., et al. (2023). 3D microperfusion of mesoscale human microphysiological liver models improves functionality and recapitulates hepatic zonation. Acta Biomaterialia. doi.org/10.1016/j.actbio.2023.09.022