According to a study published in the Proceedings of the National Academy of Sciences, Northwestern scientists discovered how cytoskeletal proteins influence the growth of developing eggs in fruit flies. These findings advance the field’s understanding of how egg cells form and distinguish themselves from other sister cells.
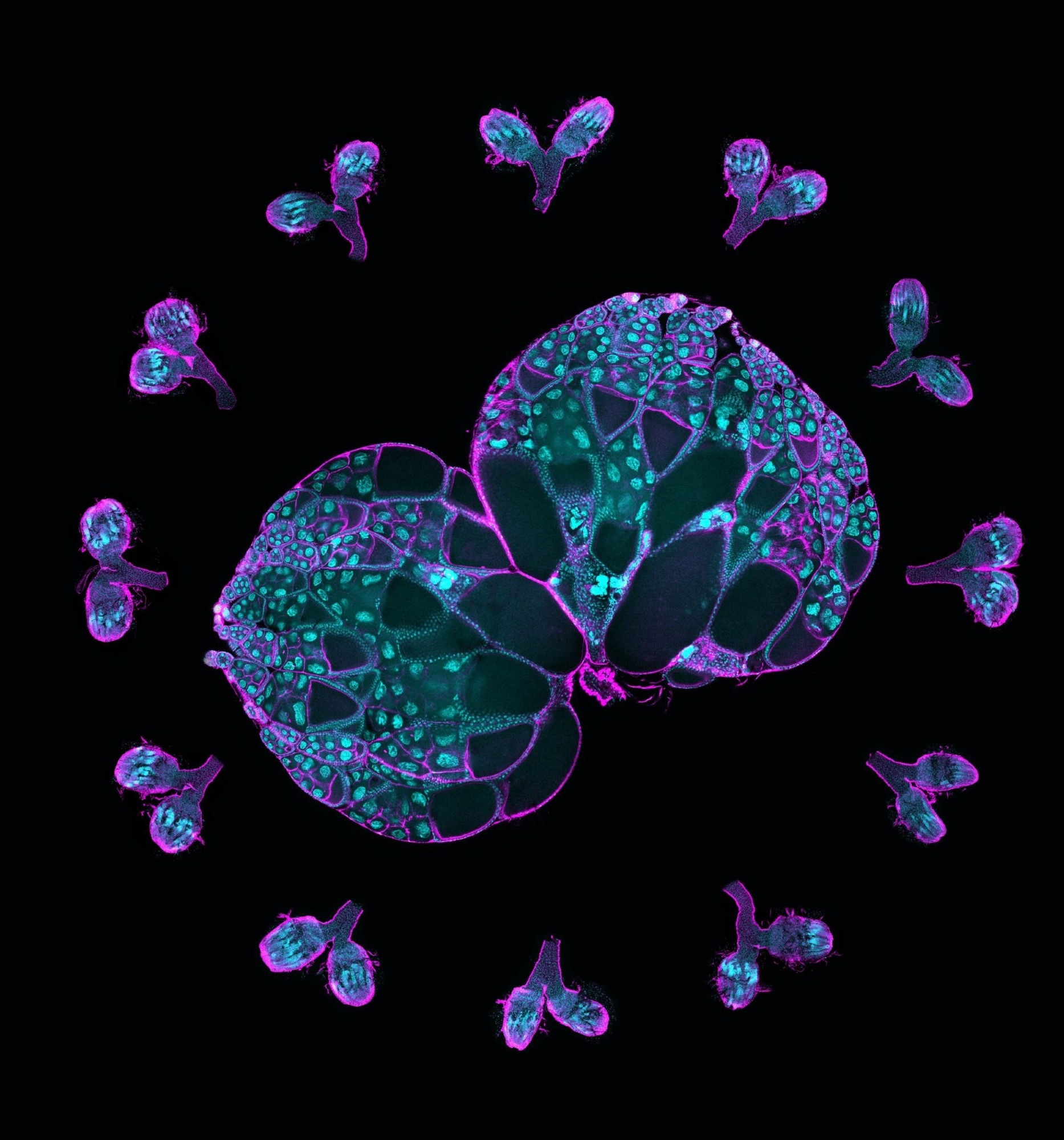 A pair of wild-type ovaries surrounded by ovaries with Msps knockdown. This image illustrates the crucial role of Msps in oocyte development. Magenta, F-actin labeling; cyan, DNA labeling. Image Credit: Gelfand Laboratory
A pair of wild-type ovaries surrounded by ovaries with Msps knockdown. This image illustrates the crucial role of Msps in oocyte development. Magenta, F-actin labeling; cyan, DNA labeling. Image Credit: Gelfand Laboratory
Oocytes, or developing eggs, are created from a group of sister cells within an ovary when one cell develops into an oocyte and the others become “nurse” cells, giving it “building materials,” such as mRNAs, proteins, and organelles. The nurse cells nourish the egg before dying off as part of the development process, according to Wen Lu, Ph.D., research assistant professor of Cell and Developmental Biology and the study’s first author.
We call this ‘winners take all,’ and these interconnected cellular structures are highly conserved; you can see this happen all the way from fruit flies, our model system, to mammals, including human beings.”
Wen Lu, Ph.D., Study First Author and Research Assistant Professor, Cell and Developmental Biology, Northwestern University
However, the processes behind this process remained unknown, prompting an additional investigation by the laboratory of Vladimir Gelfand, Ph.D., the Leslie B. Arey Professor of Cell, Molecular, and Anatomical Sciences and senior author of the study.
Essentially these oocytes are parasites. hey are not making proteins; everything is done in the nurse cells and pumped into the oocyte along microtubules, these tiny cargo tracks, by a molecular motor cytoplasmic dynein.”
Vladimir Gelfand, Ph.D., Leslie B. Arey Professor, Cell, Molecular, and Anatomical Sciences, Northwestern University
Scientists cultivated ovaries from fruit flies, which share around 75% of their DNA with humans and go through similar cellular development stages.
The researchers then employed live imaging to follow proteins within the oocyte and nurse cells. They discovered that an oocyte produced more microtubules, which are intercellular “highways” utilized to access and transfer proteins and other resources from nurse cells.
In humans, the protein XMAP215 stimulates microtubule formation, whereas mini spindles (Msps) control this process in fruit flies.
The researchers next discovered that Msps mRNA (messenger RNA), which contains the instructions for producing Msps protein, was concentrated in the oocyte cell. According to the study, mRNA knockdown had a detrimental impact on microtubule formation and cell proliferation.
Researchers discovered that dynein, a cytoskeletal motor protein that carries cargo along microtubules in cells, was responsible for the accumulation of Msps mRNA within the oocyte using single-molecule fluorescence in situ hybridization.
As per the study, removing dynein reduced the quantity of mRNA within the oocyte, which halted normal cell growth.
According to Gelfand, the findings establish Msps and dynein as a dynamic pair in directing oocyte specialization and development. Dynein transports additional Msps mRNA into the oocyte, and that mRNA generates more Msps protein, which forms more microtubule tracks for the dynein motor. According to Gelfand, dynein and Msps generate a positive feedback loop that promotes oocyte growth.
Gelfand added, “Oocyte identity is a key question of developmental biology. Knowing what is involved in defining the oocyte out of the 16 interconnected cells it is born from is important because it is the very first stage of development. It is helpful to know that microtubules and microtubule motors are a key part of that.”
Future research will concentrate on the elements that influence whether cells develop into oocytes, stated Gelfand.
Gelfand concluded, “We want to know the mechanism behind this. Is the transport of mini spindles to a cell enough to define an oocyte? If we pump it into a cell that is not supposed to be an oocyte, would that be enough to convert it? Using optogenetics, we will try and tip the scales and convert the cell’s fate.”
Source:
Journal reference:
Lu, W., et al. (2023). The dynamic duo of microtubule polymerase Mini spindles/XMAP215 and cytoplasmic dynein is essential for maintaining Drosophila oocyte fate. PNAS. doi.org/10.1073/pnas.2303376120