Reviewed by Danielle Ellis, B.Sc.Oct 16 2023
What if it was possible to heal organ damage simply by generating a new organ in the lab? Improving researchers’ capacity to print living cells on demand into geometrically well-defined, soft complex 3D structures is critical for such work and animal-free toxicological testing.
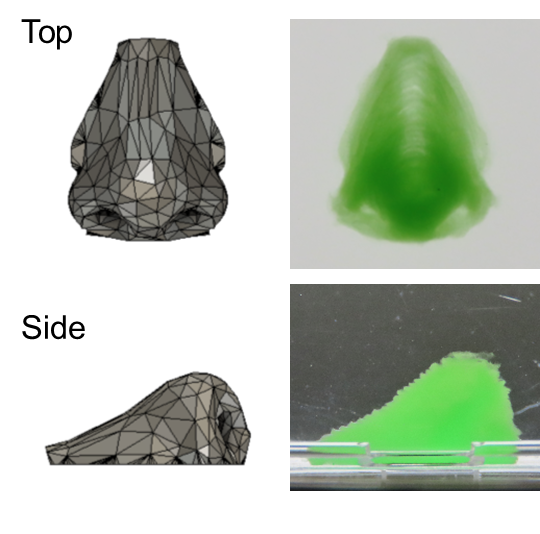 Human nose structure 3D printed with a support material. Original content, Shinji Sakai, No modifications of the work are permitted. Image Credit: Osaka University
Human nose structure 3D printed with a support material. Original content, Shinji Sakai, No modifications of the work are permitted. Image Credit: Osaka University
Osaka University researchers have overcome previous obstacles that have hampered cell proliferation and the geometrical accuracy of bioprinted designs in a study recently published in ACS Biomaterials Science and Engineering. This research might help 3D-printed cell structures get closer to imitating organic tissue and organs.
Researchers have been exploring the possibility of this layer-by-layer tissue construction technique to regenerate injured body parts and test medical theories since it was initially published in 1988 using a conventional inkjet printer.
To build 3D structures, a cell-containing “ink” is ejected from a printing nozzle in bioprinting. Hard structures are typically easier to print than soft structures. Soft structures, on the other hand, are ideal for cell proliferation in printed structures.

Image Credit: Alexander Raths/Shutterstock.com
When printing soft structures, using a printing support is useful; but, if the support is filled with ink and placed in a vessel, the ink can solidify there and get contaminated with the support’s undesirable elements. The objective of this effort was to solidify ink into a soft matrix without contamination while maintaining cell viability.
In our approach, a 3D printer alternately dispenses the cell-containing ink and a printing support. The interesting point is that the support also plays a role in facilitating the solidification of the ink. All that’s necessary for ink solidification is in the support, and after removing the support, the geometry of the soft printed cell structures remains intact.”
Takashi Kotani, Study Lead Author, Osaka University
An enzyme in the ink started to gelate as a result of hydrogen peroxide from the support, and after a few seconds, a cell assembly was surrounded in gel. The assembly was not contaminated during creation because to the quick gelation. Simple 3D creations like inverted trapezium geometries and human nose shapes—including bridges, holes, and overhangs—were easily created once the support was removed.
We largely retain mouse fibroblast cell geometry and growth, and the cells remain viable for at least two weeks. These cells also adhere to and proliferate on our constructs, which highlights our work’s potential in tissue engineering.”
Shinji Sakai, Study Senior Author, Osaka University
This novel method represents a significant advance in the engineering of human cell assemblies and tissues. To strengthen the artificial tissue's likeness to natural structures, more effort could involve better refining the ink and support as well as adding blood vessels. This work and future advancements in the exact precision of bioprinting will be helpful for regenerative medicine, pharmaceutical toxicity, and other fields.
Source:
Journal reference:
Kotani, T., et al. (2023). Horseradish Peroxidase-Mediated Bioprinting via Bioink Gelation by Alternately Extruded Support Material. ACS Biomaterials Science and Engineering. doi.org/10.1021/acsbiomaterials.3c00996