Expression of a cellular protein starts in the cell’s nucleus, the headquarters, where the cell’s coding DNA resides and where this DNA is transcribed into RNA, the first step of protein synthesis.
Transfection of protein-coding plasmid DNA into eukaryotic cells (cells found, for example, in animals, fungi, plants) is used to express a protein of interest. This technique has been used for decades but is challenged by the fact that plasmids are only transiently expressed.
Our most recent publication showed that most of the incoming plasmid is quickly caught in a cytoplasmic container, which we have named the “exclusome.” It appears in human tissue culture cells (like HeLa – one of the oldest, and most used cell lines), but it also forms in primary human fibroblasts.
We also found that if DNA reached the nucleus, it was efficiently expelled. What little DNA reached the nucleus was likely transcribed there and thus responsible for the intended plasmid-born protein expression. Expulsion removes the plasmid from the compartment where its transcription occurs, explaining the transient nature of its expression.
Curiously, the exclusome also contains DNA parts that have been shed off by the chromosomes as circles. The exclusome, therefore, appears to be a container collecting circular extra-chromosomal DNA in the cell, away from the chromosomal DNA, which is and remains in the nucleus.
Moreover, we found that as soon as plasmid DNA enters the cytoplasm, it is collected into the exclusome. This exclusome contains extra-chromosomal DNA and is surrounded by a double membrane, forming an envelope. Formation of this envelope recapitulates the early steps of nuclear envelope assembly, but the later ones.
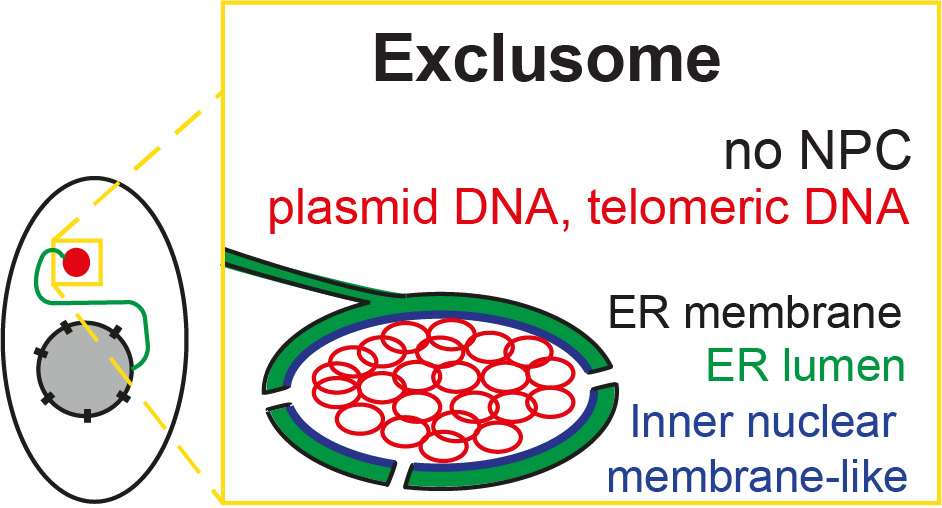 Model of Exclusome. Image Credit: Kroschewski Group
Model of Exclusome. Image Credit: Kroschewski Group
A new compartment in mammalian cells is likely to come as a surprise to many of us; what inspired this investigation?
What we found here is new because, so far, only very few people have asked where extra-chromosomal DNA goes in a cell. This extra-chromosomal DNA is sort of a mistake, something that should not be there. People were not asking where it is once it is in a cell.
We started to detect the basis of the exclusome in our previous work. Here, we discovered that plasmid DNA molecules clustered in the cytoplasm – a gelatinous liquid inside of a cell – into predominantly one structure per cell.
Moreover, in a controlled manner, the plasmid DNA molecules were asymmetrically partitioned into only one of the two resulting child cells at the end of mitosis. In the current study, we characterized this plasmid cluster and identified from where it came.
Why is the “exclusome” so exciting for our understanding of mammalian cells?
The exclusome is exciting for two reasons: first it persists for a long time – in our study, we found it survived 144 hours post-transfection. Yet importantly, it is rare to find in a cell population this many hours after transfection, as the cell is undergoing mitosis and asymmetrically partitioning during this period of time.
For example, assuming a cell cycle of 24 hours, the one cell receiving plasmid DNA at t=0 is the progenitor of about 800 cells at t=144 hours. Due to the asymmetric partitioning in theory only 1 of these 800 cells would contain an exclusome. This means that there is genetic diversity (heterogeneity) generated in that cell population.
Heterogeneity might be important for the survival of a part of that cell population if there are new environmental conditions (e.g. heat, cold, or irradiation). One could imagine that the DNA stored in the exclusome gains, under such conditions, access to the nucleus, becomes expressed and supports the survival of the given cell, in contrast to the cells without the exclusome.
This persistence also raises the possibility that there might be constant signaling originating from an exclusome to other cells in the form of cytokines. Such continual signals could be the basis of inflammatory diseases.
Second, what we learned about the envelope is exciting. As the formation of the exclusome envelope undergoes the first but not the later steps of nuclear envelope formation, we speculate that these early formation steps represent an ancient mechanism to react to any non-nuclear DNA.
Eukaryotes evolved long ago from a symbiosis between a protobacterium and an archaebacterium, which required coordination of their DNAs. As a consequence, it is possible that the nuclear envelope was established also to distinguish “self-DNA” from any other type of DNA.
Such a specialized kind of envelope would establish only one “headquarters compartment” that determines the physiology of the cell. How the distinction was made between the different types of DNA, the self and the non-self is still yet to be discovered.
 Mammalian cell with organelles. Image Credit: Soleil Nordic/Shutterstock.com
Mammalian cell with organelles. Image Credit: Soleil Nordic/Shutterstock.com
What role might it have had in the evolution of other organelles? Does the exclusome include any similar structural aspects?
The envelope of the exclusome (mentioned earlier) and that of the nuclear envelope of human cells are related. The exclusome envelope appears to have been arrested at an early stage of the formation of the nuclear envelope. This suggests either that the nuclear envelope evolved from the exclusome envelope or that the exclusome envelope is the result of an aborted nuclear envelope assembly.
It is also important to keep in mind that evolution also contributed the endoplasmic reticulum (ER) - a dynamic structure that serves many functions in a cell such as calcium storage and protein and lipid synthesis - from which both envelopes are fed. The envelopes of the exlusome and the nucleus are both specialized regions of the ER. We then ask the question: Is it possible that ER existed before the nucleus and the exclusome?
When a nuclear envelope is formed at the end of mitosis in mammalian cells, first several ER-tubes approach the DNA that was just transported during anaphase – the point when replicated chromosomes are split and the newly-copied chromosomes are moved to opposite halves of a cell.
These ER membrane patches do not fully cover the chromosomal DNA, leaving some chromosomal DNA areas uncovered (fenestrations). Within minutes, these fenestrations are sealed, whereby also nuclear pore complexes (NPCs) are inserted. This step is missing during the formation of the exclusome envelope, which can also happen during interphase.
Due to the absence of NPCs in the exclusome envelope, the Lamin B receptor protein is not recruited to the exclusome envelope and other NPC-dependent processes are assumed to not occur, such as transcription.
Described as a “container”, this new organelle is hypothesized to be a signaling hub, acting as an immune-like response to exogenous DNA. Could you explain this hypothesis to our readers?
DNA in the cytoplasm is recognized by a dedicated DNA-sensor protein, called cGAS (cyclic GMP-AMP synthase), which initiates cytokine production in the mammalian cell. Such cytokine is subsequently secreted by the cell and signals inside the multicellular organism that there is an infection.
As we showed that the exclusome with its DNA inside persists for a long time in the cytoplasm of a cell, the origin of such a signaling cascade persists and, therefore, it is possible that cytokines are produced as long as the exclusome exists. This is what we call a signaling hub. A persisting high cytokine level is a symptom of the pre-state of systemic Lupus Erythematosus, an autoimmune disease.
It is unknown whether or not the dedicated DNA-sensor protein is actually active in an exclusome, even though it appears to be present.
What are some other possible functions of the exclusome?
We can imagine that it serves as a memory for a subsequent bacterial or viral infection or general DNA encounter: The presence of the exclusome might allow a more efficient response in a second infection.
For example, the cell with an exclusome might keep the defence system for the chromosomes activated, allowing for a more rapid expulsion from the nucleus if a second DNA invasion occurs.
We can also envision it as a safety-seat belt: the exclusome seems to collect and store and silence extra-chromosomal circular DNA, but not chromosomes. Aside from mitochondria and the nucleus, it is a new DNA storage compartment. Since eliminating DNA is potentially detrimental to a cell, the cell seems to keep the collected extra-chromosomal DNA rather than eliminate it. It is still unknown under which circumstances it would be used and how.
Perhaps, there is no function for the exclusome. Instead, it could simply be a result of an old, evolutionary reaction to DNA, a default reaction.
 Image Credit: Ustyna Shevchuk/Shutterstock.com
Image Credit: Ustyna Shevchuk/Shutterstock.com
Did the team encounter any challenges in conducting this investigation? How were they overcome?
It was challenging to perform and analyse the live-cell imaging experiments to reveal the dynamics of plasmid DNA inside the cells. Laura Schenkel, the Ph.D. student in my group, who discovered the nuclear eviction of plasmid DNA, persistently improved the conditions and solved the problems that came up until a set of four suitable experiments was available. As we understood more of what was happening, she refined her analysis pipeline accordingly.
What future studies do you hope to see in this area of research?
We would like to discover how the nucleus sorts DNA to eliminate the extra-chromosomal DNA and keep the chromosomal one.
Finally, we want to know what actually happens to the incoming DNA. Is it modified? If so, how?
Where can our readers go to stay up to date with the research?
https://bc.biol.ethz.ch/research/barral/kroschewski-group.html
About Dr. Ruth Kroschewski
 Ruth started her academic career as an immunologist, before transitioning to cell biology. Thereafter, she first worked on unconventional myosins and then on Rho-GTPases in cell polarity. The later, Ruth moved to in vitro organogenesis, which is connected to asymmetric cell division, and finally, she ended up studying circular DNA.
Ruth started her academic career as an immunologist, before transitioning to cell biology. Thereafter, she first worked on unconventional myosins and then on Rho-GTPases in cell polarity. The later, Ruth moved to in vitro organogenesis, which is connected to asymmetric cell division, and finally, she ended up studying circular DNA.