Headed by James Umen, Ph.D., a team of international researchers at the Donald Danforth Plant Science Center has uncovered a surprising revelation about the single-celled green alga Chlamydomonas and its control over cell division.
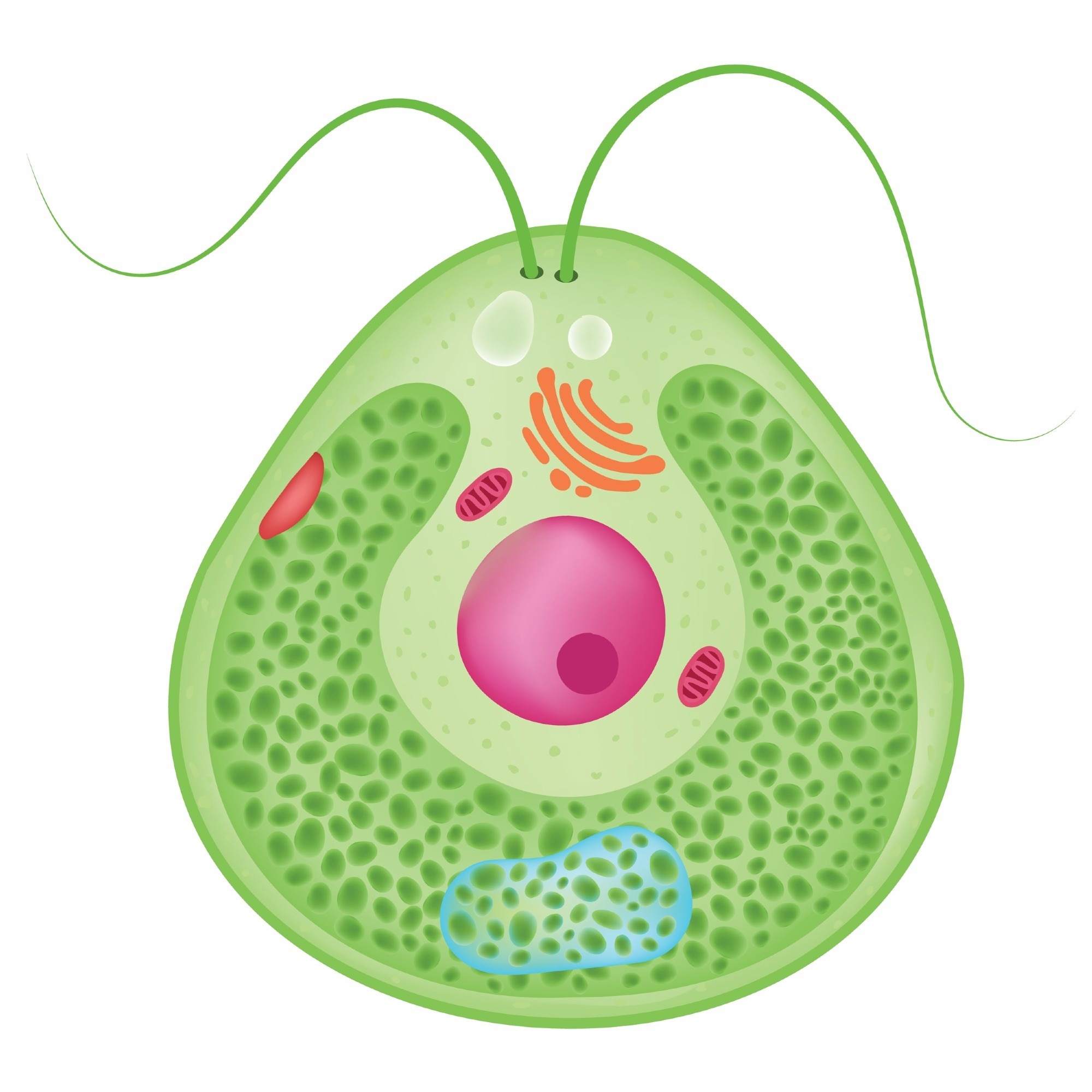
Image Credit: Achiichiii/Shutterstock.com
Contrary to the assumed mathematical optimum dictating the process, the researchers discovered a biased counting mechanism employed by Chlamydomonas. When mother cells aim to restore their daughters to the correct starting size, they exhibit a deviation from the expected pattern of divisions.
Rather than opting for a single division, mother cells tend to either refrain from dividing altogether or undergo two or more divisions.
This unexpected bias against a solitary division holds significant implications for comprehending the evolution of multicellular life. It opens up a new avenue for engineering algal cells to enhance biofuel yields and the production of high-value products.
The findings were published in Current Biology on November 9th, 2023.
Chlamydomonas cells, akin to those in various algae and single-celled protists, have the capacity to grow considerably before undergoing division. While this atypical growth and division pattern optimally utilizes light and nutrients, it poses a challenge in size control.
Depending on environmental conditions, cells may barely double in size before dividing once or, under favorable circumstances, grow more than 10 times their initial size, necessitating multiple successive divisions to generate daughters of the correct size.
This variability in size prompted the evolution of a mechanism in Chlamydomonas, enabling cells to assess their size accurately and determine the appropriate number of cell divisions.
It was always assumed that the division pattern was dictated by a simple relationship between mother cell size and number of divisions, and models that assume this simple relationship can accurately predict the behaviors of entire cell populations. But by looking at division behaviors of thousands of individual cells of varying sizes we found an unanticipated dearth of cells dividing just once.”
James Umen Ph.D., Member, Donald Danforth Plant Science Center
Contrarily, cells that were expected to undergo a single division opted to abstain from division altogether, and the majority of cells only initiated division after doubling in size or more.
Confronted with this unexpected outcome, members of the team, including Abhyudai Singh, Ph.D., a Professor at the University of Delaware, and César Augusto Vargas-García, Ph.D., Analytics Team Leader at AGROSAVIA-Corporación colombiana de investigación agropecuaria in Bogotá, Colombia, utilized mathematical modeling to develop a more precise predictive model for cell behavior.
Simultaneously, the research team at the Danforth Center, led by Dianyi Liu, Ph.D., a postdoctoral associate, delved deeper into understanding the genetic mechanisms responsible for the observed counting bias.
Their investigation revealed that the retinoblastoma tumor suppressor pathway, a well-known but still inadequately understood genetic mechanism that regulates cell division in algae, plants, and humans, played a pivotal role in preventing a singular division.
While we are just at the start of understanding how the retinoblastoma pathway works in algae, the discovery of a mechanism for introducing bias in cell division behavior immediately suggests a way that cells modified their division behavior as an important step in the evolution of multicellular life.”
Dianyi Liu Ph.D., Postdoctoral Associate, Donald Danforth Plant Science Center
The multicellular counterparts of Chlamydomonas not only bypass the possibility of undergoing a single division but also have the capability to delay division until they have significantly increased in size.
This unique trait allows a single cell to swiftly generate an entirely new multicellular individual consisting of hundreds or even thousands of cells—a crucial ability for ensuring fitness and survival.
The bias against one division we observed in Chlamydomonas was very likely present in direct ancestors of its multicellular relatives and was further amplified as they evolved greater size and complexity.”
James Umen Ph.D., Member, Donald Danforth Plant Science Center
While the reason for the evolutionary bias against a solitary division remains unclear, the understanding of this mechanism and its genetic regulation holds practical significance in algal biotechnology. Cell size can profoundly affect yields of high-value products and influence the susceptibility of algae to predation by filter feeders in open pond cultures.
Looking ahead, the team is currently concentrating on comprehending and modeling the specific mechanisms employed by the retinoblastoma pathway to modify cell division behavior in algae.
This research has the potential to advance algal biotechnology and may also provide insights into how the retinoblastoma pathway safeguards human cells against cancer development and prevents plant cells from dividing inappropriately in terms of timing and location.
Source:
Journal reference:
Liu, D., et al. (2023) A cell-based model for size control in the multiple fission alga Chlamydomonas reinhardtii. Current Biology. doi.org/10.1016/j.cub.2023.10.023.