Reviewed by Danielle Ellis, B.Sc.Dec 6 2023
Healthcare-associated infections pose a widespread challenge in the treatment of suppurating wounds, compounded by the escalating prevalence of multi-drug resistant bacteria.
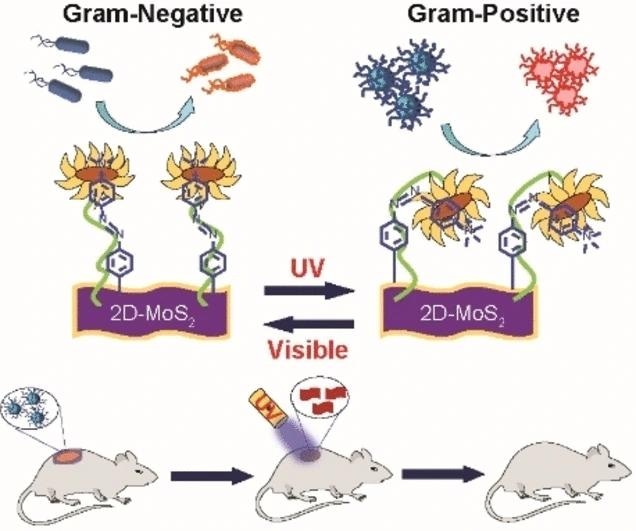
Image Credit: Angewandte Chemie.
Addressing this issue, a research team has devised a bactericidal nanomaterial featuring a photochemical “light switch” capable of selectively targeting either Gram-positive or Gram-negative bacteria.
Their findings, detailed in a study published in Angewandte Chemie, highlight the material's efficacy not only against MRSA but also its potential application in addressing various selective bacterial infections.
The urgency surrounding antibiotic-resistant infections, particularly within healthcare facilities, has reached a critical level. Many of the implicated bacterial strains are naturally occurring but can lead to severe and, at times, incurable infections in immunocompromised individuals.
In response, the development of bactericidal materials offers a novel approach to combating healthcare-associated infections without relying on traditional antibiotics.
Researchers, led by Mrinmoy De at the Indian Institute of Science in Bengaluru, India, have achieved a breakthrough by creating a UV-visible-light-responsive nanomaterial capable of switching its target specificity between Gram-positive and Gram-negative bacteria.
Image Credit: Kateryna Kon/Shutterstock.com
Gram-positive and Gram-negative bacteria exhibit distinct outer membrane structures and compositions. For instance, Gram-positive bacteria, including the notorious MRSA, feature a membrane primarily composed of peptidoglycans.
In contrast, Gram-negative bacteria, such as the problematic Pseudomonas aeruginosa, possess both inner and outer membranes primarily composed of phospholipids with a thin peptidoglycan layer.
It is important to achieve strain-selective bactericidal activity."
Mrinmoy De, Indian Institute of Science
De emphasizes the importance of achieving strain-selective bactericidal activity. To accomplish this, the research team designed a functionalized nanomaterial using molybdenum disulfide (MoS2) with attached azobenzene moieties and positively charged quaternary amino groups.
MoS2 serves as a bactericide, the quaternary amino groups induce membrane depolarization, and the azobenzene moieties introduce a light-driven switch, transforming the nanostructure from an elongated trans form to a curved cis form, facilitating selective surface interactions.
Through the use of chemical probes and optical measurements, the team identified that both cis and trans forms of the nanomaterial exhibited bactericidal activity, albeit through different mechanisms.
The trans form proved effective against Gram-negative P. aeruginosa by depolarizing the bacterial membrane and inducing thorough piercing, leading to the generation of intracellular reactive oxygen species and bacterial elimination.
Conversely, the Gram-positive MRSA strain responded more effectively to the cis form, resulting in damage and rupture of the cell wall through specific interactions.
The team demonstrated the controllable selectivity of their nanomaterial by “flipping” the UV switch from the trans ground state to the cis state. In a mouse model, the nanomaterial successfully healed MRSA-infected wounds within 10 days when treated with the cis reagent, outperforming the conventional antibiotic treatment with vancomycin.
Source:
Journal reference:
Sahoo, J., et al. (2023) Photo-Controlled Gating of Selective Bacterial Membrane Interaction and Enhanced Antibacterial Activity for Wound Healing. Angewandte Chemie International Edition. https://doi.org/10.1002/anie.202314804