Reviewed by Danielle Ellis, B.Sc.Dec 11 2023
Viruses operate with limited genetic material and a sparse protein repertoire, requiring efficient utilization of their components. The Zika virus serves as an illustrative case, producing only 10 proteins.
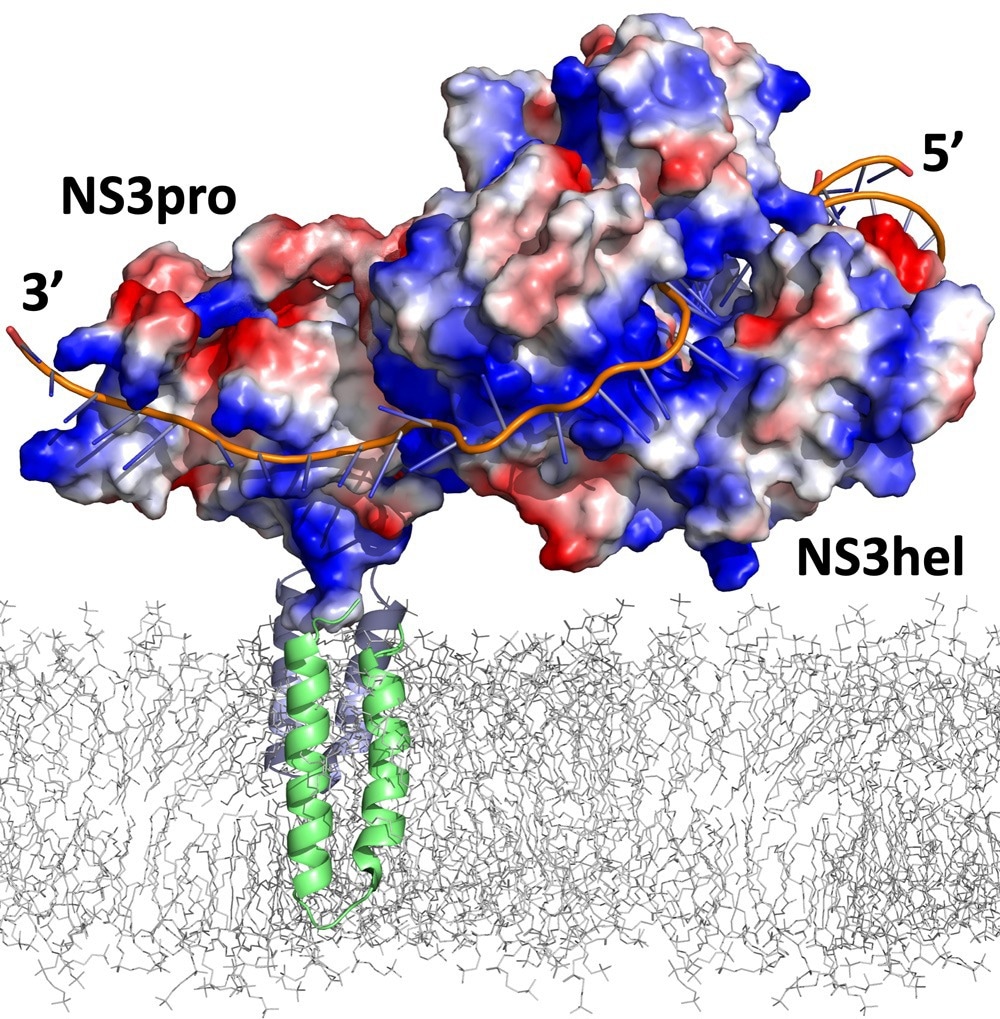 A computer model of NS2B-NS3(pro+hel) complex bound to the single strand RNA. The complex is anchored into the ER membrane (grey mesh) via NS2B helices (green in the foreground and blue in the background). Image Credit: Sanford Burnham Prebys.
A computer model of NS2B-NS3(pro+hel) complex bound to the single strand RNA. The complex is anchored into the ER membrane (grey mesh) via NS2B helices (green in the foreground and blue in the background). Image Credit: Sanford Burnham Prebys.
A recent publication in the journal PLOS Pathogens details a study conducted by researchers at Sanford Burnham Prebys, shedding light on how the virus achieves substantial functionality with its minimal resources and potentially revealing a therapeutic vulnerability.
The research team demonstrated that Zika’s enzyme, NS2B-NS3, functions as a versatile tool with dual critical roles: acting as a protease to break down proteins and serving as a helicase to separate its own double-stranded RNA into single strands.
We found that Zika’s enzyme complex changes function based on how it’s shaped. When in the closed conformation, it acts as a classic protease. But then it cycles between open and super-open conformations, which allows it to grab and then release a single strand of RNA—and these functions are essential for viral replication.”
Alexey Terskikh PhD, Study Senior Author and Associate Professor, Sanford Burnham Prebys
Zika belongs to the RNA virus family, specifically the flaviviruses, which includes other dangerous pathogens such as West Nile, dengue fever, yellow fever, Japanese encephalitis, among others. Mosquitoes transmit the virus, which targets uterine and placental cells, making it particularly perilous for pregnant women. Once inside host cells, Zika reshapes them to enhance its own replication.
Exploring Zika’s molecular intricacies could yield significant benefits, potentially identifying a therapeutic target. Targeting the domains of the enzyme responsible for protease or helicase functions poses challenges, as human cells harbor similar molecules.
However, blocking Zika's conformational changes could be an effective strategy. Without the ability to shape-shift, the virus cannot perform critical functions, preventing the production of new Zika particles.
An Efficient Machine
Zika’s essential enzyme comprises two units: NS2B-NS3pro and NS3hel. NS2B-NS3pro functions as a protease, cleaving long polypeptides into Zika proteins. Recently discovered abilities of NS2B-NS3pro include binding to single-stranded RNA and assisting in separating double-stranded RNA during viral replication.
In the study, researchers utilized recent crystal structures and employed techniques such as protein biochemistry, fluorescence polarization, and computer modeling to unravel NS2B-NS3pro’s life cycle.
NS3pro, connected to NS3hel (the helicase) by a short amino acid linker, becomes active in its closed conformation. The RNA binding occurs when the complex is open, and the transition through the super-open conformation is necessary to release RNA.
Conformational changes are driven by the dynamics of NS3hel activity, extending the linker and eventually causing the NS3pro to release RNA. NS3pro is anchored to the host cell’s endoplasmic reticulum (ER), a crucial organelle guiding cellular proteins to their destinations. In the closed conformation, NS3pro cuts the Zika polypeptide, facilitating the generation of all viral proteins.
Across the linker, NS3hel performs the task of separating Zika’s double-stranded RNA and seamlessly transfers a strand to NS3pro. The latter is equipped with positively charged “forks” that securely latch onto the negatively charged RNA.
There’s a very nice groove of positive charges. So, RNA just naturally follows that groove. Then the complex shifts to the closed conformation and releases the RNA.”
Alexey Terskikh PhD, Study Senior Author and Associate Professor, Sanford Burnham Prebys
As NS3hel extends forward to seize the double-stranded RNA, it drags the entire complex along.
However, due to NS3pro being anchored in the ER membrane and the limited extension capacity of the linker, the complex abruptly transitions into the super-open conformation, releasing RNA. Subsequently, the complex returns to the open conformation, poised for a new cycle.
Simultaneously, when NS3pro identifies a viral polypeptide to cleave, it compels the complex into the closed conformation, transforming it into a protease. The researchers term this process the “reverse inchworm” because the act of grabbing and releasing the single-stranded RNA resembles inchworm movements but in reverse, with the jaws (the protease) trailing behind.
Beyond offering a potential therapeutic target for Zika, this in-depth comprehension could be extrapolated to other flaviviruses that share similar molecular machinery.
Versions of the NS2B-NS3pro complex are found throughout the flaviviruses. It could potentially constitute a whole new class of drug targets for multiple viruses.”
Alexey Terskikh, PhD, Study Senior Author and Associate Professor, Sanford Burnham Prebys
Source:
Journal reference:
Shiryaev, S. A., et al. (2023) Dual function of Zika virus NS2B-NS3 protease. PLOS Pathogens. doi.org/10.1371/journal.ppat.1011795.