Reviewed by Danielle Ellis, B.Sc.Dec 12 2023
Non-alcoholic fatty liver disease (NAFLD), characterized by an excess accumulation of hepatic lipids, is highly prevalent and can progress to cirrhosis and liver cancer.
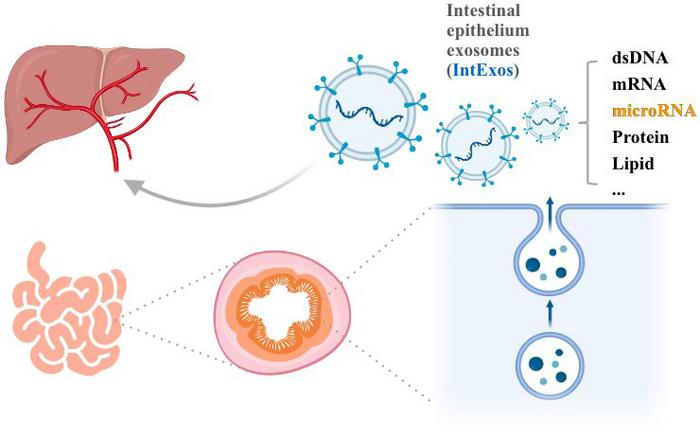 Scientific hypothesis: intestinal epithelium-derived exosomes regulate hepatic metabolic activities by carrying substances such as microRNA. Image Credit: Tiange Feng, Yuan Liang, Lijun Sun, Lu Feng, Jiajie Min, Michael W. Mulholland, Yue Yin, Weizhen Zhang.
Scientific hypothesis: intestinal epithelium-derived exosomes regulate hepatic metabolic activities by carrying substances such as microRNA. Image Credit: Tiange Feng, Yuan Liang, Lijun Sun, Lu Feng, Jiajie Min, Michael W. Mulholland, Yue Yin, Weizhen Zhang.
A comprehensive exploration of the regulatory mechanisms of NAFLD is crucial for enhancing preventive and therapeutic strategies.
Given the intimate connection between the intestine and the liver, numerous substances that regulate hepatic metabolism are either produced or absorbed by the intestinal tract. Exosomes derived from intestinal tissues have been identified as influencers of receptor cell biological processes through their cargo, suggesting a potential role in regulating hepatic lipid homeostasis.
In a recently published study in Life Metabolism scientists from Peking University affirmed that exosomes derived from intestinal epithelial cells (intExos) serve as innovative mediators in the regulation of hepatic lipid metabolism. They provided insights into partial mechanisms, particularly focusing on microRNA.

Image Credit: SewCreamStudio/Shutterstock.com
Results
To evaluate the scientific hypothesis that “intestinal epithelium-derived exosomes regulate hepatic lipid metabolism,” fluorescence tracking techniques were employed to confirm the uptake and enrichment of intExos by liver cells.
Villin-Cre;Lgr4flox/flox (VL) mice, resistant to high-fat diet (HFD)-induced fatty liver due to genetic editing in intestinal epithelial cells, were selected as the study model. IntExos collected from VL and control mice were administered to hepatic primary cells and HFD-fed mice through tail-vein injection.
The experimental outcomes consistently indicated a reduction in lipid deposition in liver cells treated with VL exosomes.
MicroRNA sequencing of intestinal epithelial exosomes from VL and control mice unveiled a significant increase in miR-21a-5p and a substantial decrease in miR-145a-5p levels in the VL group. Confirmation through overexpressing or silencing these microRNAs in liver primary cells and HFD-fed mouse livers demonstrated that miR-21a-5p alleviates NAFLD, while miR-145a-5p exacerbates hepatic lipid degeneration.
Potential target genes of miR-21a-5p and miR-145a-5p, mediating their effects on lipid metabolism, were identified using prediction databases and validated through dual-luciferase reporter gene assays.
Subsequent cell experiments revealed that miR-21a-5p downregulates C-C motif chemokine ligand 1 (Ccl1) in liver macrophages, inducing an anti-inflammatory phenotype and indirectly reducing lipid deposition in hepatocytes.
On the other hand, miR-145a-5p inhibits BTG anti-proliferation factor 1 (Btg1), leading to an increase in downstream molecules such as stearoyl-CoA desaturase-1 (Scd1) and subsequent lipid accumulation.
Conclusion
While previous research on gut exosomes has primarily focused on their roles in local conditions such as inflammatory bowel disease or the systemic influence of exosomes from feces related to the intestinal microbiota, this study addresses a critical gap by uncovering the uptake of small intestine epithelium-derived exosomes by the liver.
It elucidates the impact and mechanisms of their cargo miR-21a-5p and miR-145a-5p on hepatic lipid metabolism and inflammatory responses.
The assertion that “exosomes serve as a pathway for the intestine to regulate liver lipid homeostasis” offers new insights and feasible targets for future prevention and treatment of liver metabolic disorders.
Source:
Journal reference:
Feng, T., et al. (2023) Regulation of hepatic lipid metabolism by intestine epithelium-derived exosomes. Life Metabolism. doi.org/10.1093/lifemeta/load044.