Reviewed by Danielle Ellis, B.Sc.Jan 22 2024
In nature, the majority of bacteria adhere to a minimalistic lifestyle. When faced with nutrient scarcity or stress, they initiate a controlled shutdown of their metabolism, entering a resting state. In this dormant mode, specific metabolic processes persist, allowing microbes to sense their environment and respond to stimuli, while growth and division remain suspended.
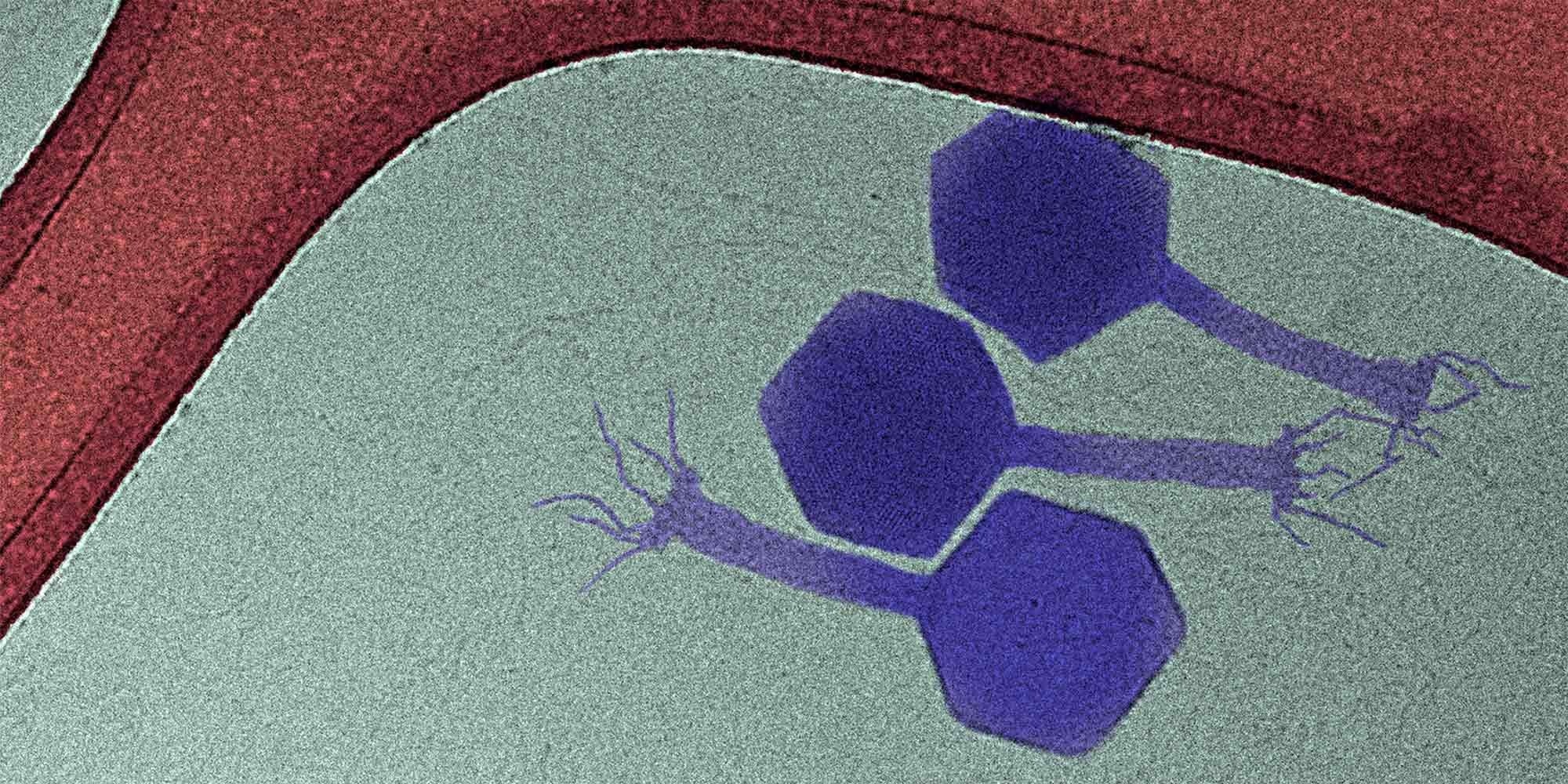 The paride phage (purple) is one of the few phages to attack dormant bacteria. Image Credit: Fabienne Estermann & Enea Maffei / ETH Zurich.
The paride phage (purple) is one of the few phages to attack dormant bacteria. Image Credit: Fabienne Estermann & Enea Maffei / ETH Zurich.
This state of dormancy serves as a defense mechanism, shielding bacteria from various threats, such as antibiotics or viruses that exclusively target bacteria, known as phages. Phages have been considered a potential alternative to antibiotics, especially when the latter become less effective due to drug resistance. Conventionally, it was widely accepted among experts that phages could only successfully infect bacteria during their active growth phase.
Researchers at ETH Zurich pondered whether evolution might have given rise to bacteriophages specializing in dormant bacteria, presenting a potential avenue for targeted interventions. Commencing their exploration in 2018, their recent publication in the journal Nature Communications affirms the existence of such phages. While these phages are uncommon, the research demonstrates their capability to target dormant bacteria.
A Lucky Strike in a Compost Heap
In 2018, when Professor Alexander Harms and his team at the Biozentrum of the University of Basel initiated their project, the initial expectation was to isolate approximately 20 different phages targeting dormant bacteria within the first year.
Contrary to this assumption, it was not until 2019 that Harms' Doctoral Student, Enea Maffei, successfully isolated a novel, previously undiscovered virus. This virus, discovered in decomposing plant material from a cemetery near Riehen in the Canton of Basel-Stadt, has the capability to infect and eliminate dormant bacteria.
“This is the first phage described in the literature that has been shown to attack bacteria in a dormant state,” Maffei says.
Harms notes, “In view of the huge number of bacteriophages, however, I was always convinced that evolution must have produced some that can crack into dormant bacteria.” They have named their new phage Paride.
Active Against Widespread Bacteria
The discovered virus targets Pseudomonas aeruginosa, a bacterium prevalent in various environments, including bodies of water, plants, soil, and the human body. In humans, certain strains of P. aeruginosa can lead to severe respiratory diseases like pneumonia, with potentially fatal consequences.
The mechanism by which the new phage catches dormant P. aeruginosa off guard remains unclear to the researchers. They hypothesize that the virus employs a specific molecular key to rouse the bacteria and then commandeers the cell's replication machinery for its own reproduction. However, the exact workings of this process are yet to be elucidated by the ETH researchers.
Therefore, their objective is to uncover the genes or molecules responsible for the awakening mechanism. By doing so, they aspire to develop substances in a laboratory setting that can initiate the wake-up process. Once identified, such a substance could be combined with a suitable antibiotic, resulting in the complete eradication of the bacteria.
But we’re just at the beginning. The one thing we know for sure is that we know almost nothing.”
Alexander Harms, Professor, ETH Zurich
Initial Tests Show an Effect
To evaluate the effectiveness of the Paride phage, researchers combined it with an antibiotic named meropenem. Meropenem disrupts cell wall synthesis, affecting only cellular processes that do not harm the phages, as dormant bacteria do not synthesize a new cell wall.
In cell culture experiments, the virus successfully eliminated 99% of dormant bacteria but left 1% unaffected. Only the combined application of Paride phages and meropenem achieved complete eradication of the bacterial culture, even though meropenem alone showed no detectable impact.
In an additional experiment conducted with Nina Khanna, a Doctor at Basel University Hospital, Maffei tested the combination of Paride phages and meropenem on mice with a chronic infection. Individually, neither the phage nor the antibiotic exhibited significant efficacy in the mice. However, the synergistic interaction between phages and antibiotics proved to be highly effective in living organisms as well.
“This shows that our discovery is not just a laboratory artifact, but could also be clinically relevant,” Maffei says.
A Glimmer of Hope – But Never More Than That?
Experts have engaged in extensive discussions on phage therapy for many years, with researchers and physicians expressing the hope that phages could eventually serve as replacements for antibiotics that have lost their effectiveness. However, widespread applications are still limited, given the absence of comprehensive studies in this field. “What we have at present is mostly individual case studies,” Harms adds.
Studies conducted by researchers at the Queen Astrid Military Hospital in Brussels revealed that the treatment improved the condition of three-quarters of patients, and it successfully eliminated the bacteria in 61% of cases. However, this implies that in four out of ten patients, the germs could not be eradicated through phage therapy despite the bacteria being phage-sensitive in laboratory conditions.
“This may be because many bacteria in the body are in a dormant state, especially in the case of chronic infections, and so phages can’t penetrate them,” Harms notes. Dormant bacteria may also contribute significantly to infections involving non-resistant strains.
In the case of infections, that means it would be important to know the physiological state of the bacteria in question. Then the right phages, combined with antibiotics, could be used in a targeted manner. However, you need to know exactly how a phage attacks a bacterium before you can select the right phages for a particular treatment. This hasn’t happened yet because we still know too little about the phages.”
Alexander Harms, Professor, ETH Zurich
In the coming years, researchers will delve into the specific mechanisms by which the new phage rouses bacteria from a dormant state, infects them, and renders them susceptible to antibiotics. This research is supported by an SNSF Starting Grant awarded to Alexander Harms and by NCCR AntiResist.
Source:
Journal reference:
Maffei, E., et al. (2024). Phage Paride can kill dormant, antibiotic-tolerant cells of Pseudomonas aeruginosa by direct lytic replication. Nature Communications. doi.org/10.1038/s41467-023-44157-3.