It is only a few centimeters in size and can be held between two fingers, but in the micro-channels carved inside it, it’s hidden a three-dimensional and highly faithful model of a biliary tract cancer called cholangiocarcinoma, complete with its tumor microenvironment. This 3D model is built starting from a sample of patient’s cancer cells and thus it represents a patient-specific "organ-on-chip": a technology made possible only through a multidisciplinary approach that merges biomedicine, physics and engineering.
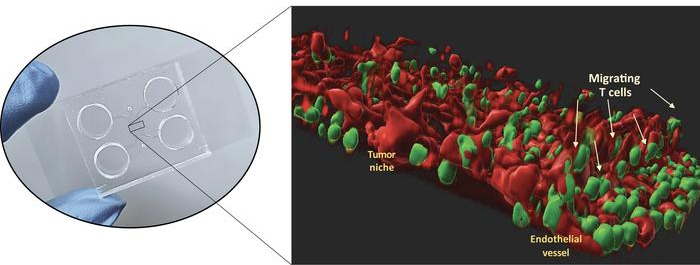 Inside the microfluidic chip, a 3D model replicates the microenvironment in which the tumor thrives, including the endothelial cells and the T cells derived from the patient. Image Credit: Politecnico di Milano
Inside the microfluidic chip, a 3D model replicates the microenvironment in which the tumor thrives, including the endothelial cells and the T cells derived from the patient. Image Credit: Politecnico di Milano
The innovative prototype is the result of the collaboration between Ana Lleo De Nalda, full professor at Humanitas University and head of the Hepatobiliary Immunopathology Laboratory at Humanitas Research Hospital, and Marco Rasponi, associate professor at Politecnico di Milano and head of the Laboratory of Microfluidics and Biomimetic Microsystems. The study was made possible thanks to the collaboration with the group of Prof. Guido Torzilli, Director of the Department of General Surgery and head of the Hepatobiliary Surgery Unit of the IRCCS Istituto Clinico Humanitas.
"The ultimate goal of the device is to accelerate research on cholangiocarcinoma by providing a new laboratory model that better mimics what we observe in patients. At the same time, it will help advancing precision medicine, since it could be potentially used as a personalized drug-testing platform, helping predict patients’ response to therapies," say Ana Lleo and Marco Rasponi.
The study was funded by AIRC - the Italian Foundation for Cancer Research, and was published in the Journal of Hepatology Reports.
Cholangiocarcinoma: A Rare and Aggressive Cancer
Cholangiocarcinoma is a rare cancer of the liver (it affects about 5,500 people in Italy alone, each year) and it derives from a malignant transformation of cholangiocytes, the cells lining the biliary tract.
Unfortunately, the disease is often diagnosed at an advanced stage, because patients show very few symptoms. This is also why treatments are often ineffective: at the time of diagnosis, only 10-30% of patients are eligible to undergo surgical removal of the tumor.
"Precisely because of the reduced therapeutic options and high mortality of cholangiocarcinoma, we need new in vitro models that can recapitulate the characteristics of the disease and in particular the interaction between tumor cells and cells of the immune system, which play a key role in its progression and response to drugs," explains Ana Lleo.
Now, for the first time, researchers from Humanitas and Politecnico di Milano developed a personalized 3D model of the disease.
A 3D Platform for Advancing Research and Personalized Medicine
"It is a microfluidic chip a few centimeters in size. Inside the device, in the micrometer channels realized using advanced photolithographic techniques, we seeded cancer cells sampled from patients affected by cholangiocarcinoma. The cells successfully reproduced the tumor architecture in vitro,” explains Marco Rasponi.
To add complexity to the model and improve its reliability, researchers seeded also fibroblasts (cells forming the connective tissue), T lymphocytes (a type of immune cells) and endothelial cells (cells lining the blood vessels that feed the cancer and screen it from immune responses).
In a series of experiments, the team of researchers demonstrated that the device faithfully recapitulates what we observe in individual patients, both in terms of T-cell activation, that correlates with tumor infiltration, and in terms of therapeutic response to different drugs, based on the characteristics of cancer recurrence.
"The next steps will be to further optimize and improve the device, both as a research model and as a personalized drug-testing platform: we want to add cells of innate immune system, such as macrophages, which play an important role in tumor progression, and introduce micro-pumps that can mimic blood flow and vascularization. We also need to test it on larger groups of patients, to confirm its ability to recapitulate the phenomena we observe in the clinical setting," conclude Ana Lleo and Marco Rasponi.