Reviewed by Danielle Ellis, B.Sc.Jan 24 2024
By tracking when distinct subsets of brain cells activate, researchers have understood the complex activity patterns found in both human and animal brains for many years. Knowing how long those neurons stay active and when they turn off again is crucial to understanding the brain and its associated disorders.
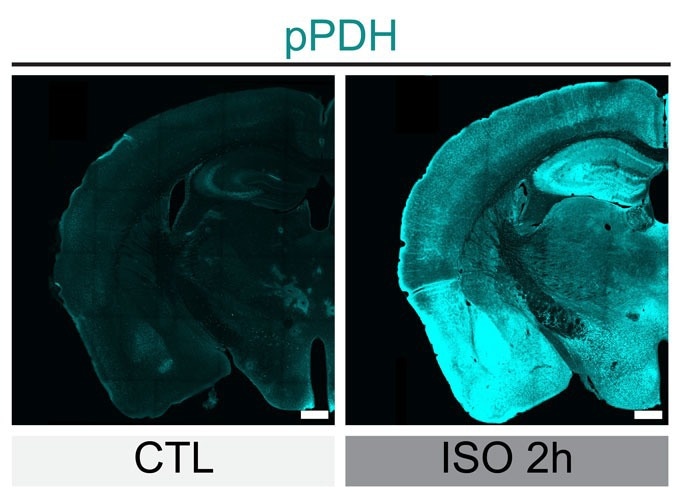 Levels of phosphorylated PDH (green) drastically increased in mouse brains cells after the mice were anesthetized for two hours (on right), showing the inhibition of the neurons. Image Credit: Scripps Research
Levels of phosphorylated PDH (green) drastically increased in mouse brains cells after the mice were anesthetized for two hours (on right), showing the inhibition of the neurons. Image Credit: Scripps Research
Now, researchers at Scripps Research have created a new tool that allows them to monitor the process of inhibition, which occurs when brain cells stop working following a spike in activity.
The method, which was published in Neuron on January 23rd, 2024, offers a new avenue for researching how the brain functions normally, as well as how illnesses and disorders like depression, post-traumatic stress disorder, and Alzheimer's disease may cause the brain's "off switches" to malfunction.
It is generally agreed that the inhibition of neurons is really the major way the brain is regulating activity, scientists have been looking for a way to look at inhibition in a more trackable way, and until now, few have found it.”
Li Ye, Professor and Abide-Vividion Chair, Scripps Research Institute
Ye collaborated with John Yates, a Scripps Research Professor of molecular medicine, to pioneer the new strategy. To better understand how brain cells differ when they are actively firing, that is when they are sending out electrical charges to communicate with their neighbors, compared to when they are not actively firing.
Image Credit: Giovanni Cancemi/Shutterstock.com
The researchers employed optogenetics, a technique that uses light to regulate a cell's activity to stimulate and inhibit the cells repeatedly. Subsequently, they quantified the various proteins' amounts, properties, and changes. They discovered that when brain cells were inhibited, pyruvate dehydrogenase (PDH), a single protein, underwent a very quick alteration.
When neurons are firing, you need a lot of energy, and this PDH protein is involved in producing that energy, but the brain really wants to conserve energy, so when a cell is done firing, we found that the brain rapidly shuts off the PDH protein. This happened much faster than anything else we saw in gene expression.”
Li Ye, Professor and Abide-Vividion Chair, Scripps Research Institute
The researchers discovered that cells tack on molecular tags to the protein called phosphates to inhibit PDH. Ye and associates discovered antibodies that were limited to identifying this phosphorylated, inactive variant of PDH (pPDH).
Ye's team measured phosphorylated PDH (pPDH) in mice that had been given anesthesia using these antibodies to see if pPDH levels might be utilized as a stand-in for brain cell inhibition. High amounts of pPDH caused nearly the whole brain to light up, accurately illustrating how most of the brain is dormant during anesthesia.
Researchers also looked at pPDH levels after bright light exposure followed by darkness for the animals. Because the active form of PDH would be needed to give these brain cells signaling energy, brain cells in the visual cortex, which is responsible for vision, showed low levels of pPDH when exposed to light. However, high levels of phosphorylated protein surged soon after the light was turned off.
Ye's group also investigated a little-known mechanism with the new method: how the brain suppresses appetite after eating. They demonstrated how, as a mouse begins to eat, brain cells linked to appetite turn off.
These discoveries may help to understand better appetite, obesity, and some weight reduction medications. More generally, brain cell inhibition levels in healthy individuals and those with a range of brain and metabolic disorders might be compared using the pPDH antibodies.
Ye said, “There are a lot of questions that this technology can help us answer: if the brain can not turn cells off, or if they are turned off faster or slower than usual, what happens? How does the inhibition of neurons play a role in different diseases?”
Source:
Journal reference:
Dong Yong., et.al., (2024) Phosphorylation of pyruvate dehydrogenase inversely associates with neuronal activity. Neuron. doi.org/10.1016/j.neuron.2023.12.015