Dibenzothiophene S-oxides are important in various biochemical processes. However, their synthesis using conventional methods is not easy. Researchers from Tokyo University of Science have now developed an innovative two-step process involving a Suzuki–Miyaura coupling of 2-bromoaryl-substituted sulfinate esters, followed by intramolecular electrophilic sulfinylation. This method can facilitate the facile synthesis of polysubstituted dibenzothiophene oxides without damaging highly reactive functional groups. The resulting compounds can find applications in fields like drug development and biochemical research.
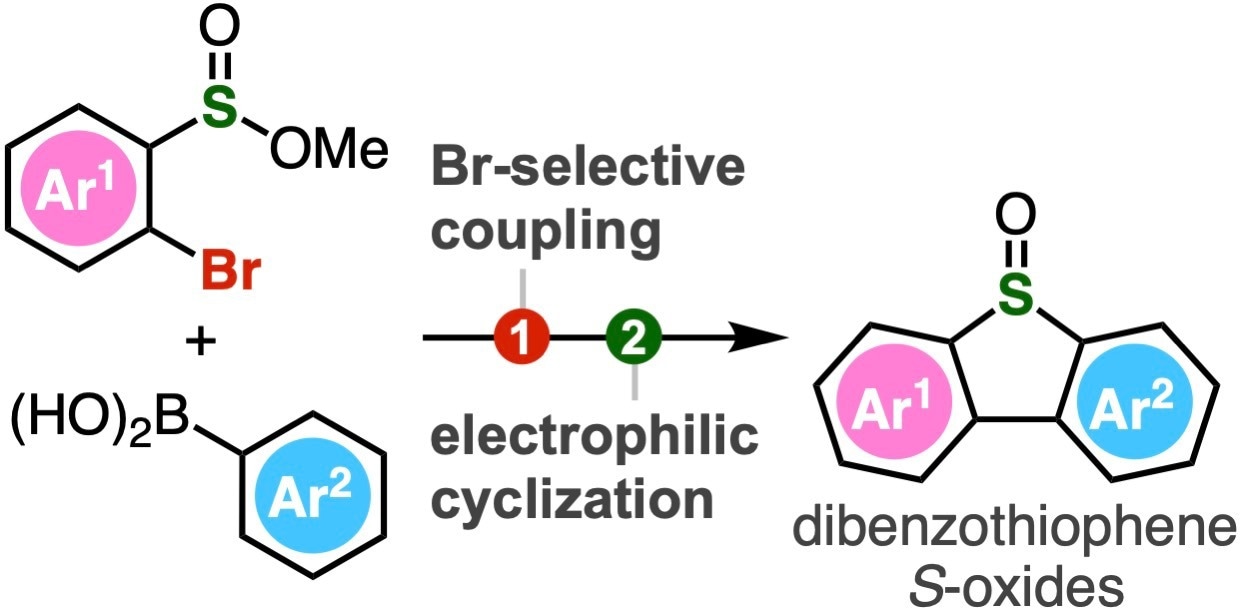 A range of polysubstituted dibenzothiophene oxides can be synthesized through Br-selective coupling and subsequent cyclization by electrophilic activation. Image Credit: Suguru Yoshida from Tokyo University of Science
A range of polysubstituted dibenzothiophene oxides can be synthesized through Br-selective coupling and subsequent cyclization by electrophilic activation. Image Credit: Suguru Yoshida from Tokyo University of Science
Organic compounds in the field of chemistry range from simple hydrocarbons to complex molecules, with diverse functional groups added to the main carbon backbone. These functional groups impart the compounds distinct chemical properties as well as participate in various chemical transformations, making them important precursors for the synthesis of diverse compounds. Scientists have, therefore, actively engaged in creating molecules that feature novel and highly reactive functional groups.
One such class of compounds are dibenzothiophenes and their derivatives containing S-oxide or S,S-dioxide moieties (sulfur atoms bonded to one and two oxygen atoms respectively). These compounds are of special interest in the fields of pharmaceutical sciences, materials chemistry, and chemical biology. Dibenzothiophenes consist of benzene rings fused to a thiophene ring—a five-membered ring with four carbon atoms and one sulfur atom. When dibenzothiophene S-oxides are exposed to UV light, they release atomic oxygen, which is useful for DNA cleavage and oxidation of adenosine-S’-phosphosulfate kinase, an enzyme involved in cellular processes. Additionally, the S–O bond can be activated to introduce different functional groups, enabling the creation of a wide range of molecules with diverse properties and applications. The conventional method of producing functionalized dibenzothiophene S-oxides involves thiophene ring formation followed by subsequent S-oxidation. However, this reaction is challenging to carry out.
To address this, Associate Professor Suguru Yoshida, Ms. Yukiko Kumagai, Mr. Akihiro Kobayashi, and Mr. Keisuke Nakamura from Tokyo University of Science (TUS) have developed a simple two-step method of synthesizing dibenzothiophene S-oxides. The method involves Suzuki–Miyaura coupling of 2-bromoaryl-substituted sulfinate esters, followed by an intramolecular electrophilic sulfinylation.
The details of the method, published in the journal Chemical Communications on 10 January 2024, opens possibilities for creating a variety of important sulfur-containing molecules in the life sciences that were traditionally difficult to synthesize using conventional methods.
“Dibenzothiophene oxides are attracting attention in the field of chemical biology, and several researchers have developed a reaction using dibenzothiophene oxide, which can now be synthesized using this method. We expect this research to elucidate life phenomena involving reactive oxygen species,” explains Dr. Yoshida, while talking about this study.
The Suzuki–Miyaura coupling is a widely used organic reaction between boronic acids and organic halides, leading to the formation of a new carbon–carbon bond. In the proposed method, sulfinate esters react first with arylboronic acids in the presence of a palladium catalyst. Next, the intermediate biaryl compounds are activated with Tf2O, leading to subsequent cyclization by electrophilic activation.
Compared to the conventional oxidation method of synthesizing dibenzothiophene, this innovative approach developed by Dr. Yoshida and his team can accommodate a wide range of functional groups, including highly reactive ones, enabling the synthesis of polysubstituted dibenzothiophene oxides not achievable earlier. Using the method, the researchers synthesized dibenzothiophene oxides having an o-silylaryl triflate moiety, a compound useful as an aryne generation site, but tends to get easily damaged when produced using conventional methods. The o-silylaryl triflate moiety serves as a useful reactive intermediate and can undergo various transformations to produce highly substituted arenes. The proposed method, therefore, not only simplifies the synthesis method but also opens doors for a diverse range of dibenzothiophene S-oxides and their derivatives.
The novel method is a significant step forward in the field of chemical biology. Going ahead, the researchers anticipate that these compounds can find useful applications in diverse research areas, paving the way for innovations and discoveries. “The proposed method can enable the synthesis of polysubstituted benzothiophene oxides, which are expected to be useful in a wide range of research fields,” concludes Dr. Yoshida.