Over the last two decades, ongoing research in microfluidics has faced challenges in applications due to the restricted productivity of microdroplets. In a recent study, researchers from Chiba University have tackled this issue by developing an efficient method to boost the speed of microdroplet generation for microfluidic devices. This was achieved by incorporating porous, inverse colloidal crystal structures into conventional microchannels, offering potential applications in medicine, food, cosmetics, and semiconductor manufacturing.
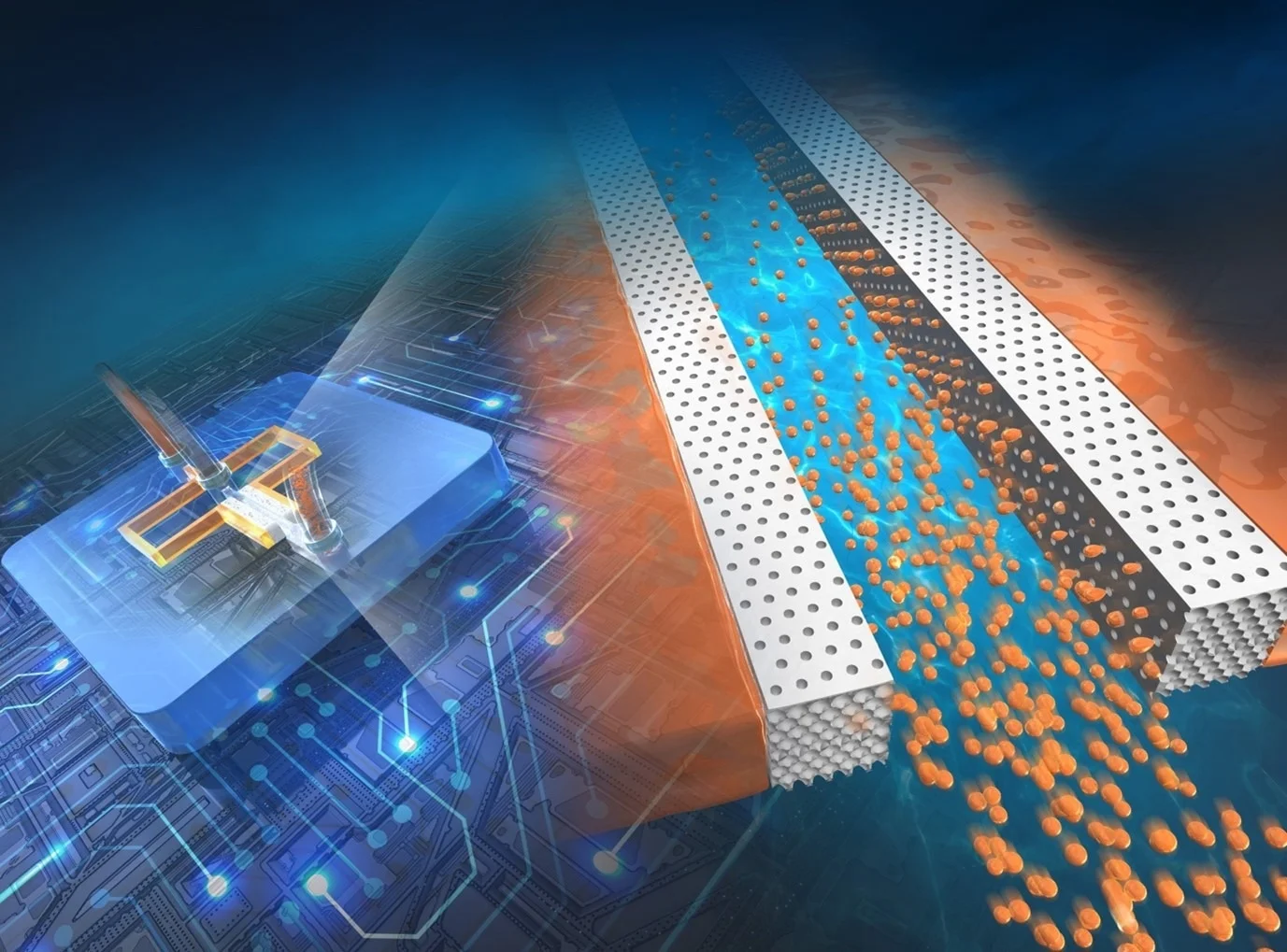 Image Credit: Chiba University
Image Credit: Chiba University
Over the past two decades, microfluidic devices, which use technology to produce micrometer-sized droplets, have become crucial to various applications. These span chemical reactions, biomolecular analysis, soft-matter chemistry, and the production of fine materials. Furthermore, droplet microfluidics has enabled new applications that were not possible with traditional methods. It can shape the size of the particles and influence their morphology and anisotropy. However, the conventional way of generating droplets in a single microchannel structure is often slow, limiting production.
In a recent study featured in the 21 January 2024 issue of the journal Lab on a Chip, led by Associate Professor Masumi Yamada from the Department of Applied Chemistry and Biotechnology at the Graduate School of Engineering at Chiba University and including Shota Mashiyama from the Graduate School of Science and Engineering, also at Chiba University, as the first author, researchers have introduced a microfluidic system that utilizes porous "inverse colloidal crystal" (ICC) structures to dramatically improve the efficiency of microdroplet generation.
"We considered that highly efficient droplet formation might be possible by using the numerous micropores formed on the surface of the ICC structure as droplet-forming nozzles. However, to the best of our knowledge, no study has been reported on the integration of inverse colloidal crystal structures into microfluidic channels and their application to highly efficient droplet formation. Therefore, we decided to develop a new microfabrication technique to integrate these structures into microfluidic channels to achieve efficient droplet formation," emphasizes Dr. Yamada.
In the study, spongy ICC structures were integrated with flat microchannels, which functioned like tiny nozzles to produce droplets around 1,000 times faster than traditional microfluidic devices. The size of the droplets could also be changed by adjusting the flow of liquids, their properties, and the size of the tiny openings. Furthermore, single micrometer-sized particles made of natural biopolymers like polysaccharides and proteins were also produced using this method. This new approach improves the existing concept of droplet microfluidics, not only by increasing the speed at which droplets are formed but also by making the process easier to create and operate.
Due to the improved efficiency and control in the formation of droplets, this new method is expected to have a broad impact across different fields and product categories. This includes medicine, food, cosmetics, specialized inks and paints, sieving matrices for bioseparation, and the creation of functional particles for displays and semiconductor applications.
"Microdroplets, biopolymer particles and vesicles fabricated from them as scaffolds, are widely used for medical applications such as drug development and regenerative medicine. Additionally, this method is expected to be applied to the production of various substances, including carriers for the controlled delivery of drugs, scaffolds for cell culture, reagents for cell transformation, carriers of antigens in cellular immunotherapy, and functional microparticles for diagnostics," envisions Dr. Yamada.
In summary, the researchers have developed a method for quickly forming droplets at an extremely high speed for microfluidic devices by integrating three-dimensional ICC structures into traditional flat microchannels. By applying this technique to produce materials for diverse purposes, it is expected to contribute to the advancement of technologies that improve people's lives and support overall well-being!