For patients with spinal cord injuries, a small gadget developed by researchers at MIT and the Singapore-MIT Alliance for Research and Technology may help to increase the efficacy and safety of cell therapy treatments.
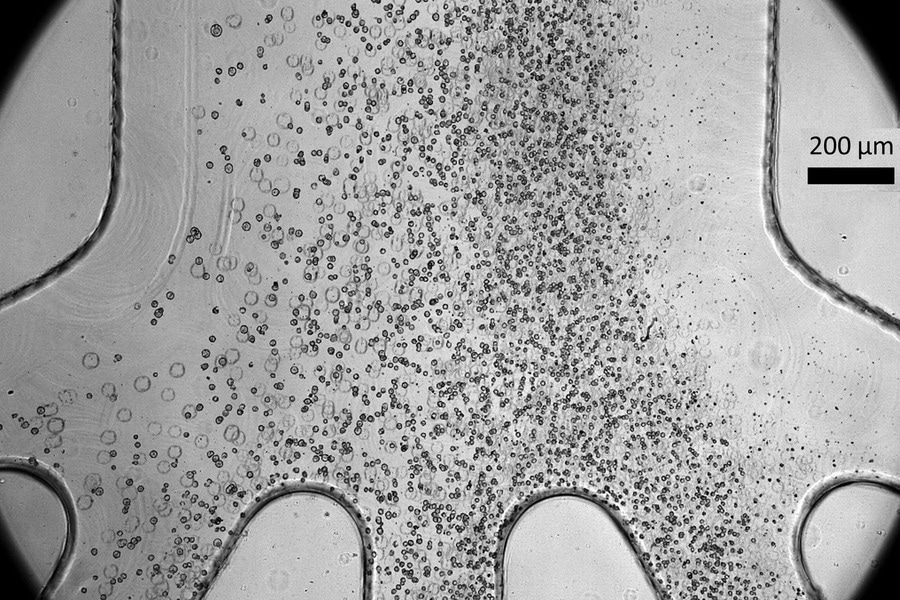 Scientists developed a tiny microfluidic device that can improve the safety and efficacy of cell therapy techniques for spinal cord injury patients. Their device, which can sort cells by size as shown in the photo, can remove a large percentage of stem cells that have not yet fully become spinal cord cells, which could potentially form tumors after being transplanted into a patient. Image Credit: Massachusetts Institute of Technology
Scientists developed a tiny microfluidic device that can improve the safety and efficacy of cell therapy techniques for spinal cord injury patients. Their device, which can sort cells by size as shown in the photo, can remove a large percentage of stem cells that have not yet fully become spinal cord cells, which could potentially form tumors after being transplanted into a patient. Image Credit: Massachusetts Institute of Technology
In cell therapy, doctors reprogram some patient skin or blood cells to produce what are referred to as induced pluripotent stem cells. These pluripotent stem cells would be encouraged to develop into progenitor cells, which will eventually differentiate into spinal cord cells, to cure a spinal cord injury. The patient then receives a second donation of these progenitors.
A portion of the damaged spinal cord can be repaired thanks to these new cells. Tumors can, however, develop from pluripotent stem cells that do not fully differentiate into progenitors.
This group of researchers created a microfluidic cell sorter that can eliminate roughly half of a batch's undifferentiated cells tumor-possible cells without endangering the progenitor cells that have fully matured.
The high-throughput device can sort over three million cells each minute without special chemicals. Furthermore, the researchers have demonstrated that connecting multiple devices in a chain can sort over 500 million cells every minute, which makes this a more practical way to enhance the safety of cell therapy treatments in the future.
Furthermore, the microfluidic cell sorter's plastic chip can be mass-produced in a factory at a relatively low cost, making it easier to implement the device on a larger scale.
Even if you have a life-saving cell therapy that is doing wonders for patients, if you cannot manufacture it cost-effectively, reliably, and safely, then its impact might be limited. Our team is passionate about that problem — we want to make these therapies more reliable and easily accessible.”
Jongyoon Han, Professor, Department of Electrical Engineering and Computer Science, Massachusetts Institute of Technology
He is also a Professor of Biological Engineering, a member of the Research Laboratory of Electronics (RLE), and a Co-Lead Principal Investigator of the CAMP (Critical Analytics for Manufacturing Personalized Medicine) research group at the Singapore-MIT Alliance for Research and Technology (SMART).
Han contributed to the paper by joining Co-Senior Author Sing Yian Chew, Professor of Chemistry, Chemical Engineering, And Biotechnology at the Lee Kong Chian School of Medicine and Materials Science and Engineering at Nanyang Technological University in Singapore and a CAMP Principal Investigator.
And co-lead authors Tan Dai Nguyen, a CAMP researcher; Wai Hon Chooi, a senior research fellow at the Singapore Agency for Science, Technology, and Research (A*STAR); and Hyungkook Jeon, an MIT postdoc; as well as others at NTU and A*STAR. The research was published in Stem Cells Translational Medicine.
Reducing Risk
One of the biggest obstacles to this kind of cell therapy is the cancer risk that undifferentiated induced pluripotent stem cells generate.
Han added, “Even if you have a very small population of cells that are not fully differentiated, they could still turn into cancer-like cells.”
Researchers and clinicians frequently use specific markers found on the surfaces of these undifferentiated cells to try and identify and eliminate them, but thus far no marker has been shown to be unique to these cells. Some approaches employ chemicals to kill these cells only, however the differentiated cells may be harmed by the chemical treatment procedures.
After over ten years of labor, the CAMP team had previously built a high-throughput microfluidic sorter that could sort cells depending on size. The technique is currently being extended to other stem cell types, such as induced pluripotent stem cells, by the team. Previously, it was employed for the sorting of immune cells and mesenchymal stromal cells, another kind of stem cell, Han explained.
Chew said, “We are interested in regenerative strategies to enhance tissue repair after spinal cord injuries, as these conditions lead to devasting functional impairment. Unfortunately, there is currently no effective regenerative treatment approach for spinal cord injuries.”
Spinal cord progenitor cells derived from pluripotent stem cells hold great promise, since they can generate all cell types found within the spinal cord to restore tissue structure and function. To be able to effectively utilize these cells, the first step would be to ensure their safety, which is the aim of our work.”
Sing Yian Chew, Co-Senior Author and Professor, Department of Chemistry and Chemical Engineering, Massachusetts Institute of Technology
The group found that pluripotent stem cells typically have a bigger size than the progenitors that they are produced from. A pluripotent stem cell's nucleus is thought to have a large number of genes that have not been repressed or switched off before the cell develops. The cell shrinks the nucleus dramatically when it develops for a particular role, suppressing many genes it will no longer need.
This size difference is exploited by the microfluidic device to sort the cells.
Spiral Sorting
The quarter-sized plastic chip contains microfluidic channels that form an input, a spiral, and four exits that produce various-sized cells. Centrifugal forces are one of the many forces acting on the cells as they are driven through the spiral at extremely high speeds.
To concentrate the cells in a specific area of the fluid stream, these forces oppose one another. The size of the cells will determine this focus point, which will efficiently sort them through different outlets.
The sorter's performance could be enhanced, the researchers discovered, by operating it twice: once at a slower pace to allow larger cells to adhere to the walls and separate from smaller ones, and again at a faster speed to separate larger cells.
The microfluidic sorter functions somewhat like a centrifuge, but Han notes that it does not require human assistance to identify sorted cells.
The scientists demonstrated how their tool could eliminate almost half of the bigger cells in a single run. To make sure that the bigger cells they had destroyed were, in fact, linked to a higher risk of tumor development, they ran trials.
While we cannot remove 100 percent of these cells, we still believe this is going to reduce the risk significantly. Hopefully, the original cell type is good enough that we do not have too many undifferentiated cells. Then this process could make these cells even safer.”
Jongyoon Han, Professor, Department of Electrical Engineering and Computer Science, Massachusetts Institute of Technology
Crucially, there is no need for filtration in the low-cost microfluidic sorter, which can be mass-produced using conventional production methods. A gadget without a filter can be operated for far longer periods of time because filters can clog or malfunction.
The researchers are starting larger investigations and animal models to investigate if the purified cells operate better in vivo now that they have demonstrated effectiveness on a small scale.
Removing more nondifferentiated cells may increase the safety and effectiveness of cell therapies since these cells can have a variety of unpredictable consequences in the body in addition to becoming cancers.
Han said, “If we can convincingly demonstrate these benefits in vivo, the future might hold even more exciting applications for this technique.”
Source:
Journal reference:
Nguyen., D. et.al., (2024). Label-Free and High-Throughput Removal of Residual Undifferentiated Cells From iPSC-Derived Spinal Cord Progenitor Cells. Stem Cells Translational Medicine. doi.org/10.1093/stcltm/szae002