Discoveries in mice could guide the discovery of treatments to address brain developmental disorders in children with SYNGAP1 gene mutations.
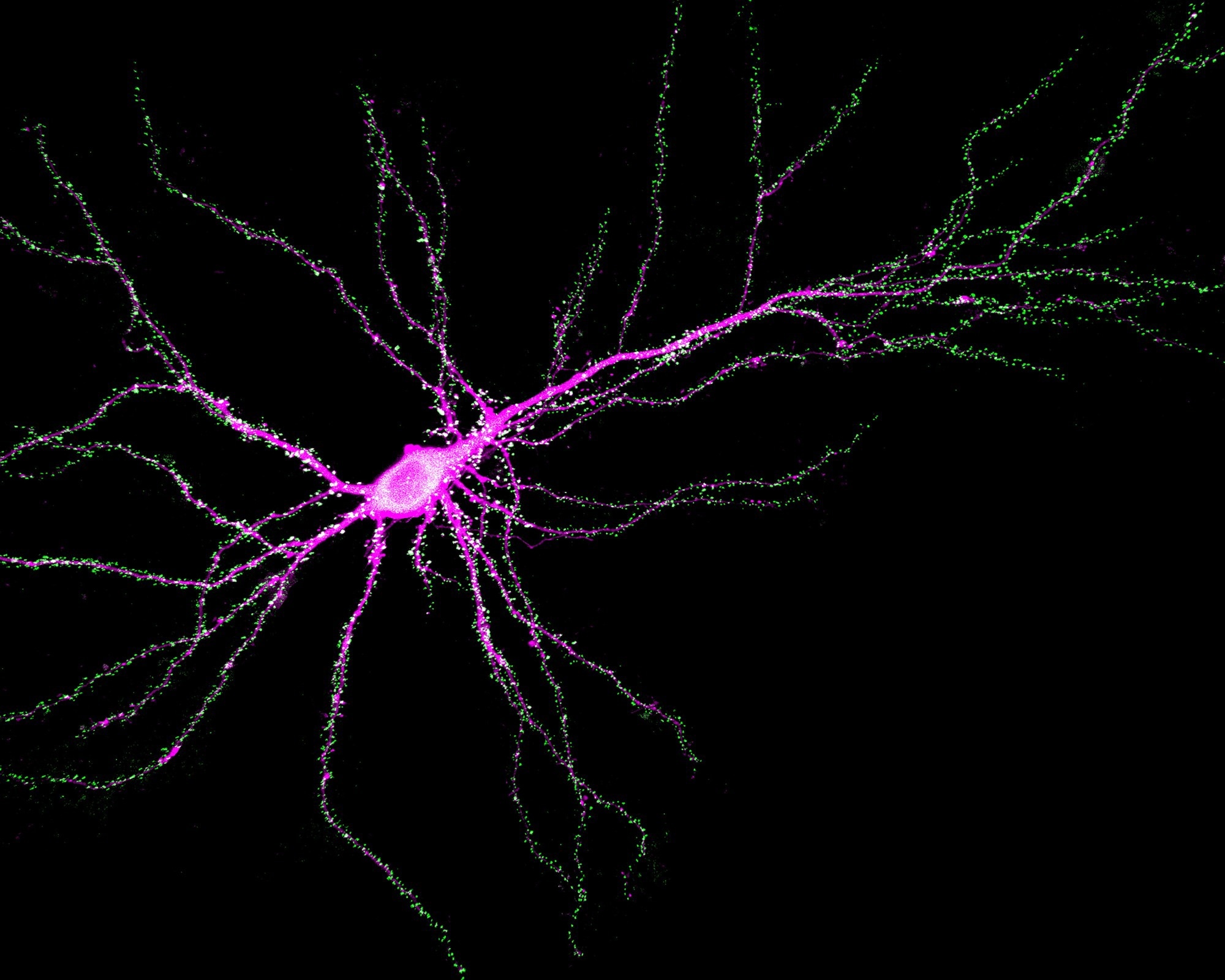 Neuron showing SynGAP (green) binding to PSD-95 at synapses. Image Credit: Yoichi Araki and Rick Huganir. Johns Hopkins Medicine
Neuron showing SynGAP (green) binding to PSD-95 at synapses. Image Credit: Yoichi Araki and Rick Huganir. Johns Hopkins Medicine
Neuroscientists from Johns Hopkins Medicine have recently discovered an additional role for the SYNGAP1 gene, a specific DNA sequence responsible for regulating memory and learning in both mice and humans.
The discovery, published on March 1st, 2024, in the journal Science, could have implications for advancing treatments aimed at children with SYNGAP1 mutations. These children experience various neurodevelopmental disorders characterized by intellectual disability, behaviors resembling autism, and epilepsy.
Typically, SYNGAP1, along with various other genes, manages the process of learning and memory through the production of proteins that oversee the synaptic strength, which are the links between neurons.
The SYNGAP1 gene was previously believed to solely encode an enzyme-like protein that controls chemical reactions influencing synaptic strength. However, recent experiments on mice conducted by scientists suggest that the protein produced by this gene may also serve as a scaffolding protein, regulating synaptic plasticity regardless of its enzymatic functions.
According to the researchers, the SynGAP protein functions as a traffic manager, determining the location and type of brain protein at synapses.
In 1998, Richard Huganir, Ph.D., a Distinguished Professor of Neuroscience and Psychological and Brain Sciences at Johns Hopkins University School of Medicine, and Huganir’s team successfully identified the SYNGAP1 gene.
Huganir states that SynGAP proteins are highly prevalent at the synapse, and it has been widely believed that the primary function of SynGAP is to initiate enzymatic reactions that control the synapse's strength.
However, through their research on the SynGAP protein, Huganir and colleagues discovered an intriguing characteristic: when SynGAP proteins come into contact with the primary synaptic scaffolding protein, PSD-95, they transform into liquid droplets.
For an enzymatic protein, that structural transformation is unusual.”
Richard Huganir, Professor, Johns Hopkins University School of Medicine
To unravel and comprehend the reason behind SynGAP’s unique liquid alteration, Huganir, neuroscience educator Yoichi Araki, and Huganir’s team of researchers at Johns Hopkins conducted trials on neurons.
They introduced mutations into the GAP domain of the SYNGAP1 gene, which would eliminate SynGAP’s enzymatic function while preserving its structure.
The team from Johns Hopkins discovered that, despite lacking enzymatic activity, the synapse operated normally. This indicates that the structural aspect alone plays a crucial role in the functioning of SynGAP.
The research team subsequently replicated the genetic engineering process in mice to eliminate the enzymatic function of SynGAP. The outcome was consistent with the previous findings: the synapses functioned normally, exhibiting no issues in synaptic plasticity, and the mice displayed no challenges in their learning and memory abilities.
Based on these observations, the research team concludes that the structural aspect of SynGAP alone is satisfactory for maintaining regular cognitive behavior.
The researchers conducted a detailed analysis of synapses to comprehend the role of SynGAP’s structure in synaptic regulation. Through their investigation, they discovered that the SynGAP protein competes with the binding of AMPA receptor/TARP complexes, which are a group of neurotransmitter proteins responsible for enhancing synapses, as well as the PSD-95 scaffolding protein.
The findings indicate that SynGAP forms a strong bond with PSD-95 when resting, preventing any interaction with other proteins within the synapse. Nevertheless, during synaptic plasticity, learning, and memory processes, the SynGAP protein dissociates from PSD-95, exits the synapse, and attaches neurotransmitter receptor complexes to PSD-95. As a result, the synapse becomes more robust, leading to enhanced communication between brain cells.
This sequence happens without the catalytic activity typical of SynGAP.”
Richard Huganir, Professor, Johns Hopkins University School of Medicine
Instead, SynGAP captures PSD-95 upon binding, but once SynGAP departs from this synapse, PSD-95 becomes available to bind with AMPA receptor/TARP complexes.
In children affected by SynGAP mutations, approximately half of the SynGAP proteins are present in the synapse. This reduction in SynGAP proteins leads to an increased binding of PSD-95 with the AMPA receptor/TARP complexes.
Consequently, this alteration in neuronal connections results in heightened brain cell activity, which is a defining feature of epileptic seizures commonly observed in children with SynGAP mutations.
According to Huganir, the significance of SynGAP's enzymatic function and its role as a scaffolding protein in “traffic management” should not be overlooked when searching for treatments for SynGAP-related neurodevelopmental disorders. Additionally, their study indicates that solely targeting one function of SynGAP may not yield substantial results.
Source:
Journal reference:
Araki, Y., et al. (2024) SynGAP regulates synaptic plasticity and cognition independently of its catalytic activity. Science. doi.org/10.1126/science.adk1291