The research community is searching for alternative bactericidal treatment approaches due to the global prevalence of drug-resistant bacteria. Japanese researchers have now compared the effectiveness of enzymes derived from bacteriophages in fighting drug-resistant bacteria.
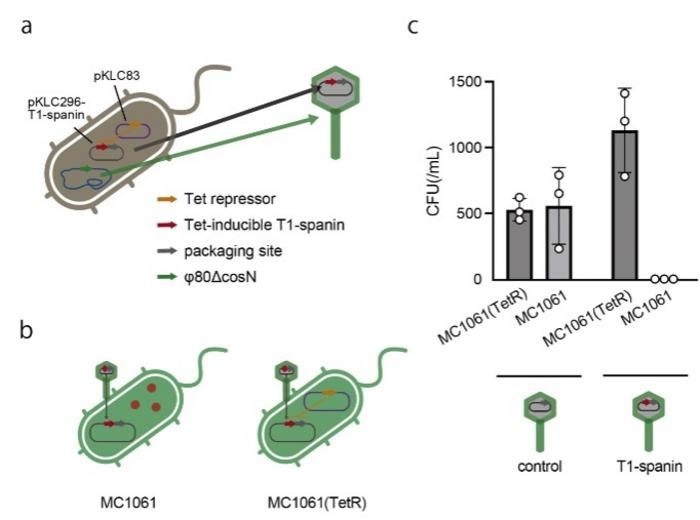 (a) Schematic diagram of the construction of a φ80 phage capsid containing T1-spanin gene. T1-spanin exhibits toxicity toward synthetic bacterium 594; therefore, its expression was suppressed using a Tet repressor-expressing plasmid (pKLC83). Due to the presence of a packaging sequence, the T1-spanin plasmid was encapsulated within a phage capsid. (b) The synthesized phage capsid (a) was used to infect Escherichia coli MC1061. Within bacterial cells lacking Tet repressor expression, T1-spanin was expressed. (c) E. coliMC1061 were infected with phage capsids containing either the T1-spanin-encoding plasmid or a plasmid lacking T1-spanin (control). The infected bacteria were plated on LB agar plates, and the number of colony-forming units (CFUs) was counted. Abbreviation: LB, Luria-Bertani. Assays were performed in triplicate. The bars show the means of the three spot test results, and the error bars show the standard deviations. Image Credit: BioDesign Research
(a) Schematic diagram of the construction of a φ80 phage capsid containing T1-spanin gene. T1-spanin exhibits toxicity toward synthetic bacterium 594; therefore, its expression was suppressed using a Tet repressor-expressing plasmid (pKLC83). Due to the presence of a packaging sequence, the T1-spanin plasmid was encapsulated within a phage capsid. (b) The synthesized phage capsid (a) was used to infect Escherichia coli MC1061. Within bacterial cells lacking Tet repressor expression, T1-spanin was expressed. (c) E. coliMC1061 were infected with phage capsids containing either the T1-spanin-encoding plasmid or a plasmid lacking T1-spanin (control). The infected bacteria were plated on LB agar plates, and the number of colony-forming units (CFUs) was counted. Abbreviation: LB, Luria-Bertani. Assays were performed in triplicate. The bars show the means of the three spot test results, and the error bars show the standard deviations. Image Credit: BioDesign Research
According to examination, T1-spanin exhibits superior bactericidal activity against various strains, including E. coli. In addition, a new phage-based technique successfully introduces T1-spanin genes into the target bacteria. This discovery could pave the way for the future creation of novel antimicrobial agents.
Global efforts have recently been directed toward addressing unprecedented and emerging health risks, like the COVID-19 pandemic. However, the ongoing threat to global public health comes from the persistence of drug-resistant bacteria. For example, in 2019 alone, drug-resistant bacteria caused approximately 1.27 million deaths globally each year.
More people die each year from antibiotic-resistant strains than from HIV and malaria put together. Even though antibiotic-resistant bacteria are still a threat to the healthcare system, other treatment modalities like bacteriophage therapy are starting to show promise.
Viruses known as “phages,” or bacteriophages, are designed to specifically target and eliminate bacteria, including ones that have developed antibiotic resistance.
Some phages are more potent than others because they contain specific enzymes that they employ in various ways to target and destroy bacteria.
One kind of enzyme that can destroy bacteria from the inside out is phage-derived lytic enzyme. By using bacteriophage therapy, scientists have now discovered a way to effectively and precisely target drug-resistant pathogens by using the power of these enzymes.
To investigate and refine this strategy of focusing on bacteria that are resistant to antibiotics, a team of Japanese researchers led by Satoshi Tsuneda and Kotaro Kiga carried out an investigation into the special characteristics of the T1 phage. The team set out to comprehend the mechanism of action of this renowned broad-spectrum agent that targets specific bacterial strains.
The results of this investigation were published in the journal BioDesign Research, and they provided insight into the possible use of T1 phage in improving bacteriophage therapy against pathogens that are resistant to drugs.
To do this, the scientists first contrasted T1 phage-derived enzymes with T7 phage-derived enzymes. An analysis was conducted to evaluate the antibacterial properties of endolysins, holins, and spanins. Alongside holins to control their activity, endolysins are known to break down the bacterial cell wall from the inside, causing the cell to rupture.
Holins work by puncturing the inner membrane of bacteria through oligomerization, with the help of a transmembrane domain. Spanins, on the other hand, act by mediating the fusion between the outer and inner membranes.”
Tsuneda Satoshi, Professor, Nanjing Agricultural University the Academy of Science
Additionally, spanins aid in the breakdown of the bacterial cell wall. The T1 phage's enzymes proved to be the most effective at killing bacteria out of all the enzymes the researchers looked at.
T1-spanin in particular was noteworthy. When viruses attack bacteria, they take control of their internal DNA machinery and use it to replicate themselves, a process that is similar to what is seen in humans.
The bacterium's cell bursts open to release the freshly formed virus particles into the surrounding environment after enough copies have been made inside. Spanins, such as T1-spanin, are crucial to this process because they aid in the breakdown of the bacterial cell membrane, which permits the virus particles to be released.
The enzyme T1-spanin demonstrated a remarkable ability to breach the outer barriers of nearly 120 distinct bacterial strains. The researchers created a unique method to directly insert the T1-spanin gene into target bacteria by using a creative approach. To do this, the T1-spanin gene had to be integrated into a template virus shell.
Unlike natural bacteriophages, this synthetic virus is unable to reproduce itself, and by using the synthetic virus instead of the bacteriophage directly, we were able to reduce the risk of environmental contamination or any adverse effects.”
Tsuneda Satoshi, Professor, Nanjing Agricultural University the Academy of Science
Unlike conventional methods, the strategy created by the researchers in this work can be used to successfully target a broad variety of bacteria because of T1-spanin's broad applicability.
This work gives hope, even though it may be hard to see how something as tiny as an enzyme produced by a virus can affect the threat posed by bacteria that are resistant to antibiotics.
It illustrates the potential of novel strategies to combat drug-resistant pathogens and advance the development of therapeutic interventions, and it suggests how molecular-level innovative strategies can help address the most pressing challenges in global public health.
Source:
Journal reference:
Yamashita, W., et al. (2024) Harnessing a T1 phage-derived spanin for developing phage-based antimicrobial development. Biodesign Research. doi.org/10.34133/bdr.0028