Scientists at the Francis Crick Institute have provided insight into the proteins governing the development of mice's ovaries both prenatally and postnatally. This might help comprehend the progression of female infertility.
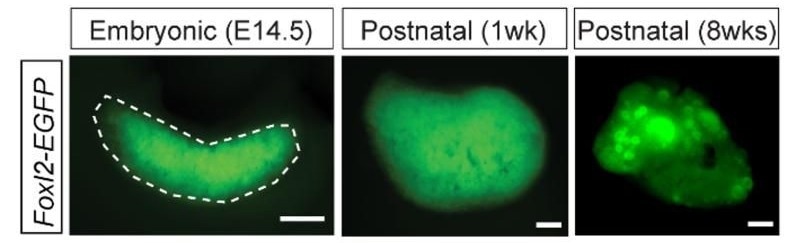 Fluorescent images which show that FOXL2 expression can be followed using a fluorescent reporter (green) added to tag the FOXL2 protein using genome editing techniques. Image Credit: Roberta Migale, Science Advances
Fluorescent images which show that FOXL2 expression can be followed using a fluorescent reporter (green) added to tag the FOXL2 protein using genome editing techniques. Image Credit: Roberta Migale, Science Advances
After discovering the gene that causes the ovaries to begin developing in the mouse embryo, researchers set out to determine which genes continue to function in the ovaries after birth, including the ability to produce eggs.
Prior research has demonstrated that the effects of deleting the Foxl2 gene in female (XX) mice vary depending on when it is done and at what stage of development. Mice that have had their embryos removed have aberrant ovaries and are sterile as adults. When taken out of adult mice, their ovaries start to look like testes.
The researchers discovered that although FOXL2 is involved in embryonic development, its effects are greatest after birth when the protein controls the activity of numerous additional genes, some of which are essential for ovarian functions like egg development. The study was published in Science Advances.
Proteins called FOXL2 physically atop particular DNA regions, or “enhancers,” control the reading of other (target) genes and how they are read.
The other proteins that interact with FOXL2 when it is bound to DNA were all "fished out" by the researchers using a method known as chromatin proteomics. When compared to the embryonic stage of development, they discovered that the number of protein interactions in ovaries increased significantly after birth.
They discovered, among many other things, that FOXL2 binds to a protein known as USP7 when it interacts with its DNA targets. The relationship between USP7 and FOXL2 or USP7's function in ovarian development was unknown to researchers until recently.
When the Usp7 gene was taken out of female mice, the researchers discovered that the mice were infertile because they were unable to develop ovaries past puberty. The researchers think that USP7 might be required to stabilize FOXL2 above DNA.
In humans, FOXL2 and USP7 play a few similar roles. Individuals with a single copy of the FOXL2 gene are able to begin ovulating, but their ovaries do not fully develop, leading to infertility. Neurodevelopmental disorders and infertility in humans are also associated with USP7 mutations.
Researchers aim to identify the primary genetic causes of infertility and explore the potential role of gene editing techniques in future treatments, as genetic testing plays a crucial role in diagnosing issues related to sexual development.
In our research, we’ve come closer to answers for two major questions regarding development - what drives ovary development, and how the function of the ovary is maintained. We’ve found that FOXL2 has very different roles throughout development and identified another crucial protein, USP7. The genetic factors underlying female development haven’t been as well studied as male development, because many female developmental pathways happen at the same time rather than in an easy-to-follow sequence. Infertility is a big problem worldwide, so shedding light on the key genes and proteins responsible at each stage is vital.”
Robin Lovell-Badge, Group Leader, Stem Cell Biology and Developmental Genetics Laboratory, The Francis Crick Institute
“This is the first time we’ve been able to use these approaches to see the interactions that FOXL2, a factor critical for female fertility, establishes with other proteins whilst they are bound to DNA in mouse ovaries. Factors that actively bind to the DNA are more likely to have an impact on the regulation of genes important for the development and function of the ovary. We’ve identified USP7 through this method and the hope is that many more proteins responsible for ovary development can be found using our approach,” added Roberta Migale, Postdoctoral Fellow at the Crick and first and co-senior author of the study.
The function of the USP7 protein in sexual development will be further investigated by the researchers.
Source:
Journal reference:
Migale, R., et al., (2024) FOXL2 interaction with different binding partners regulates the dynamics of ovarian development. Science Advances. doi.org/10.1126/sciadv.adl0788