The most challenging subtype of breast cancer to treat is triple-negative breast cancer (TNBC). Over 20,000 cases of TNBC are reported each year in the United States alone. The five-year mortality rate is approximately 40%, which is worse than the results experienced by patients with other subtypes of breast cancer.
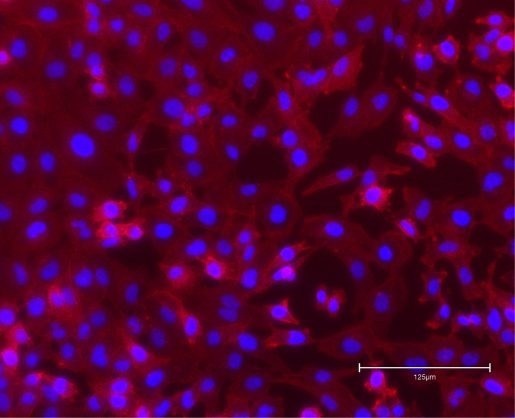 The image shows MDA-MB-231 triple-negative breast cancer cells growing on a plastic laboratory dish. Blue shows the nucleus and red visualizes the actin cytoskeleton of the cells. Image Credit: Dr. Aparna Mitra in the Foulds lab.
The image shows MDA-MB-231 triple-negative breast cancer cells growing on a plastic laboratory dish. Blue shows the nucleus and red visualizes the actin cytoskeleton of the cells. Image Credit: Dr. Aparna Mitra in the Foulds lab.
The propensity of cancer cells to metastasize or spread to other organs and the dearth of efficient cancer-specific medicines are regarded to be the causes of the high death rate.
Dr. Charles Foulds, an Assistant Professor at Baylor College of Medicine, and members of the Dan L Duncan Comprehensive Cancer Center and the Lester and Sue Smith Breast Center are working to better understand TNBC and possibly find vulnerabilities that could lead to more effective therapies.
Searching for TNBC Vulnerabilities With KIPA
In this study, published in PNAS Nexus, we searched for enzymes called kinases, whose expression is typically altered in cancer."
Dr. Junkai Wang, Study First Author, Baylor College of Medicine
Wang was also a member of the Foulds lab while he was working on this project.
Prior research has demonstrated that therapeutic targeting of kinases can be beneficial in treating different types of cancer. Numerous inhibitors of these enzymes that have previously received FDA approval for use in humans could be investigated for possible therapeutic benefit in TNBC.
“The challenge was to identify the kinase among hundreds of kinases in TNBC cells that could potentially give us an upper hand against this cancer,” Wang said.
The researchers used a laboratory method they had previously developed, called kinase inhibitor pull-down assay (KIPA), which significantly speeds up the process of kinase identification among hundreds of potential candidates.
Using 16 patient-derived xenografts—human breast cancer tumors grown in immune-compromised mouse models—the team employed KIPA to identify kinases that showed significant alterations in triple-negative breast cancer (TNBC) compared to normal cells.
We found that TNBC cells have more of the kinase called Death-Associated Protein Kinase 3 (DAPK3). We confirmed this finding in TBNC cell lines and in tumors.”
Dr. Junkai Wang, Study First Author, Baylor College of Medicine
It is crucial to emphasize that although DAPK3 protein levels were elevated in TNBC, those of its precursor mRNA were not. The mRNA molecule carries the DAPK3 gene's genetic information, which the cell uses to synthesize the protein.
Wang said, “Many studies only rely on mRNA data to assess what proteins are produced by cells. If we had just looked into mRNA and not the protein levels in TNBC, we would have missed that the DAPK3 protein is overproduced in this cancer and deserves further attention.”
A New Role for DAPK3
Subsequent research revealed that knocking down the DAPK3 gene did not alter the proliferation of cancer cells; it did stop TNBC cells from migrating and invading in lab settings.
When TNBC cells with the DAPK3 gene removed were cultured as tumors in immune-compromised mice, no substantial influence on tumor metastasis was detected. More metastasis modeling is required to provide a definitive answer.
The researchers learned more about how DAPK3 mediates its impact on migratory promotion.
“We found that DAPK3 reduces the levels of desmoplakin (DSP), a protein that is involved in regulating cell adhesion, which is related to a cell’s ability to migrate. In addition, we discovered that a protein called LUZP1 binds to DAPK3, which protects it from being destroyed by the cell, ” Wang said.
Altogether, our findings have improved our understanding of how kinases control the spread of TNBC cells. We previously thought that a driver of cancer may affect cell proliferation and migration. But we found that in TNBC, DAPK3 does not regulate growth, but controls migration and invasion. Our next steps include conducting additional studies to learn more about how the DAPK3/LUZP1 complex functions to promote TNBC migration and assess its potential value as a therapeutic target.”
Dr. Charles Foulds, Assistant Professor, Baylor College of Medicine
Source:
Journal reference:
Wang, J., et al. (2024) DAPK3 modulates migration and invasion of triple negative breast cancer cells. PNAS Nexus. doi.org/10.1093/pnasnexus/pgae401.