Researchers from EMBL Heidelberg and the University of Virginia School of Medicine have uncovered a fascinating mechanism by which cells respond to nutrient deprivation. This discovery has exciting implications for understanding cancer development.
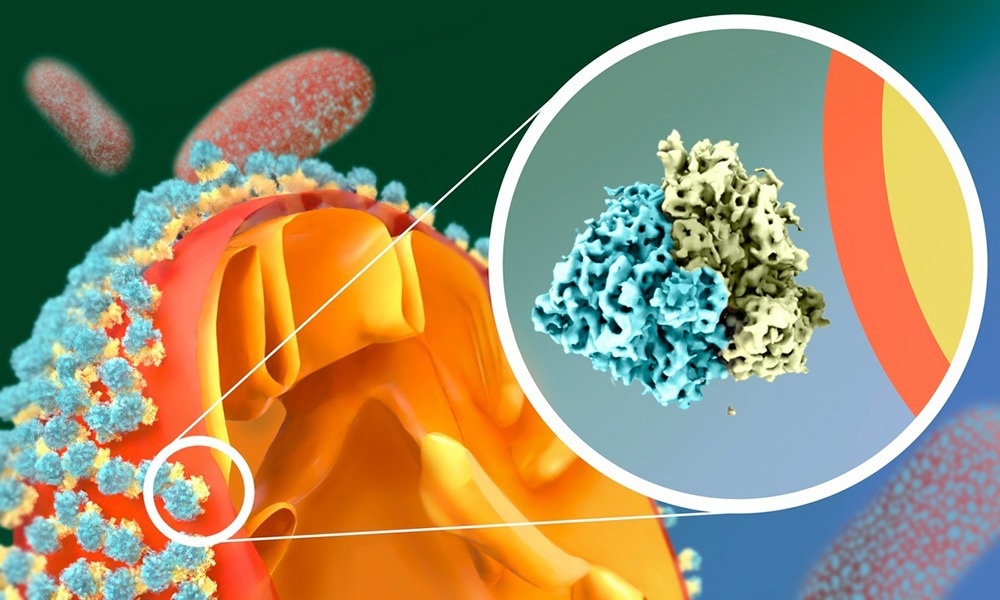 In starved fission yeast, the ribosomes attach to the mitochondrial outer membrane via their small subunit. This is a very unusual “upside-down” orientation. Image Credit: Isabel Romero Calvo/EMBL
In starved fission yeast, the ribosomes attach to the mitochondrial outer membrane via their small subunit. This is a very unusual “upside-down” orientation. Image Credit: Isabel Romero Calvo/EMBL
What insights can stressed yeast provide about fundamental cellular processes? The Mattei Team at EMBL Heidelberg says a lot. The group investigates how cells adjust to nutritional deprivation and other forms of stress.
The yeast species S. pombe, which has been utilized in traditional brewing for generations, is one of their favorite test subjects. As a eukaryote, it is in many respects identical to human cells, thus researchers commonly utilize it as a model organism to investigate fundamental cellular processes.
Ribosomes Turn Upside-Down in Hungry Cells
Researchers have noticed that yeast cells have a great way of surviving starvation: many huge molecular complexes known as ribosomes wrap their mitochondria.
This strange behavior intrigued the Mattei Team and the Jomaa Lab at the University of Virginia School of Medicine, so they used cryo-electron tomography and single-particle cryo-electron microscopy to investigate it more deeply.
Ribosomes are the cell’s heavyweight molecular machinery that generates proteins. However, it was discovered that the ribosomes that swarm the mitochondria's surface in starving yeast cells generate nothing. It is hibernation for them.
One way for a cell to survive stressful conditions until better days is to reduce its use of energy to a minimum. Producing proteins demands a lot of energy, which can be saved by blocking ribosomes.”
Olivier Gemin, EIPOD Postdoctoral Fellow and Study Lead, European Molecular Biology Laboratory
It is unknown why the dormant ribosomes adhere to the mitochondrial surface.
There could be different explanations. A starved cell will eventually start digesting itself, so the ribosomes might be coating the mitochondria to protect them. They might also attach to trigger a signaling cascade inside the mitochondria.”
Simone Mattei, Team Leader, European Molecular Biology Laboratory
Mattei is also investigating the notion that starved cells require a mechanism to start generating energy quickly when food (in the form of glucose) becomes accessible again. Since mitochondria are the cell's primary energy source, having ribosomes close by to make essential proteins may facilitate this process.
The scientists were stunned when they discovered that ribosomes adhere to the outer membrane of the mitochondria in a manner that defies prior knowledge.
“So far, ribosomes were known to interact with membranes only via their large subunit. But in starved cells, we saw that they do this upside-down, via the small subunit!” said Mattei.
In their upcoming research, the group will investigate how and why the ribosomes adhere in such an odd manner.
Cancer Cells Go Through the Hell They Create
There are parallels between cancer cells' struggles and those of the malnourished yeast cells.
Being a cancer cell is indeed incredibly difficult. When a tumor becomes aggressive, its cells proliferate so quickly that the amount of oxygen and nutrients they require cannot keep up with their needs. This implies that the majority of cancer cells live in a kind of self-created hell where they are continuously starved.
And yet they endure, even proliferating.
That is why we need to understand the basics of adaptation to starvation and how these cells become dormant to stay alive and avoid death. For that, we use yeast first, because we can manipulate it much more easily. Beyond this, we try to starve cultured cancer cells too, which is not easy, to figure out how they overcome starvation and can sometimes lead to cancer relapse.”
Ahmad Jomaa, Assistant Professor and Study Senior Co-Author, University of Virginia
Understanding the mechanisms behind this adaptation could enable one to disrupt it, making cancer cells more susceptible to starvation and, consequently, more responsive to treatment.
Source:
Journal reference:
Gemin, O., et al. (2024) Ribosomes hibernate on mitochondria during cellular stress. Nature Communications. doi.org/10.1038/s41467-024-52911-4.