Förster resonance energy transfer, or FRET for short, is a well-known microscopy method that is used to find out the proximity of a pair of fluorophores.
Resonance energy transfer occurs only across very short distances, typically within 10 nm. In this method, excited state energy is directly transferred from the donor fluorophore to an acceptor fluorophore, offering an alternative to fluorescence emissive decay caused by the donor.
Resonance Energy Transfer
Upon transferring the energy, the acceptor molecule assumes an excited state and then decays emissively and invariably at a longer wavelength as opposed to the acceptor emission. Hence, the time at which, and the level of efficiency with which the FRET occurs can be determined by exciting the donor and then tracking the corresponding donor and acceptor emissions, either sequentially or together.
Considering that the fluorophores can be used for labeling biomolecules and that the FRET’s distance condition is of the order of the diameter of a majority of biomolecules, FRET is extensively used to determine where and when two or more biomolecules communicate inside their physiological setting.
Since energy transfer occurs over a distance of 1 to 10 nm, a FRET signal corresponding to a particular location in a microscope image offers additional distance precision, and thus exceeds the optical resolution of the light microscope.
FRET Efficiency
Apart from spatial proximity, the FRET dye pair should show a substantial overlap of the excitation spectrum of the donor with the absorption spectrum of the acceptor. This ensures the efficient occurrence of FRET. But this overlap is tied to one of the experimental paradoxes of FRET.
Spectral profiles in the FRET pair cannot be properly separated, such that overlaps exist, but “cross-talk” between the two imaging channels is not preferred in general.
Preferably, the donor emission filter set must collect light only from the donor and vice versa, and not from the acceptor. As a matter of fact, this can be realized to some extent by utilizing short bandpass filters that only extract light from the shorter wavelength side of the donor emission and the longer wavelength side of the acceptor emission.
But this can slightly limit the photon flux caused by the donor and the acceptor emissions during a standard exposure, specifically when these measurements can be optimally performed under reduced excitation power conditions so that bleaching rates are not expedited. The emission and absorption spectral profiles of the CFP-YFP FRET pair are illustrated in Figure 1.
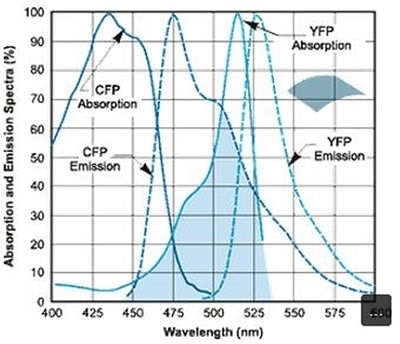
Figure 1. Absorption and emission spectral profiles of the CFP-YFP FRET pair. Image Credit: Andor Technology Ltd.
Andor Technology has developed EMCCD cameras that serve as a robust tool for FRET imaging. It can be used either as a main component in the Revolution confocal live-cell imaging system offered by Andor Technology, or as an EMCCD + iQ imaging software solution.
The EMCCD camera offers excellent resolution and allows high signal-to-noise determination of FRET interactions that occur across the area or volume of the cell that is imaged. It also helps in countering the sacrifice of photon throughput that occurs when narrow-band filters are utilized.
By integrating the EMCCD camera with appropriate filter sets, the high integrity of FRET data can be obtained.
Considering that EMCCD cameras have the potential to overcome the detection limit of noise floor at all readout speeds, molecular interactions can be monitored with excellent accuracy. Photo-bleach effects are also reduced by decreasing the excitation power and thus make it possible to track molecular interactions for a considerably longer duration of time.
Conclusion
An important topic of interest across various fields of biological research is the nature of molecular interactions that occur in living cells. But studies are usually held up because the instruments used for such analyses have limited resolution.
Through FRET measurements, the distances between biomolecules labeled with suitable donor and acceptor fluorophores can be determined, specifically when they are within 10 nm of one another.
About Andor Technology
 Andor Technology, an Oxford Instruments company, is a global leader in the pioneering and manufacturing of high performance scientific imaging cameras, spectroscopy solutions and microscopy systems for research and OEM markets. Andor has been innovating the photonics industry for over 20 years and continues to set the standard for high performance light measuring solutions, enabling its customers to break new ground by performing light measurements previously considered impossible. Andor’s digital cameras, are allowing scientists around the world to measure light down to a single photon and capture events occurring within 1 billionth of a second.
Andor Technology, an Oxford Instruments company, is a global leader in the pioneering and manufacturing of high performance scientific imaging cameras, spectroscopy solutions and microscopy systems for research and OEM markets. Andor has been innovating the photonics industry for over 20 years and continues to set the standard for high performance light measuring solutions, enabling its customers to break new ground by performing light measurements previously considered impossible. Andor’s digital cameras, are allowing scientists around the world to measure light down to a single photon and capture events occurring within 1 billionth of a second.
Andor now has over 400 staff across 16 offices worldwide, distributing products to over 10,000 customers in 55 countries. Andor’s products are used in a wide range of applications including medical research to further the understanding of heart disease, cancer and neuronal diseases such as Alzheimer’s and Parkinson’s disease. Andor also has applications for forensic science and astronomy. Through continuous dialogue with customers and strong teamwork, Andor continues to innovate ground-breaking products that improve the world in which we live.
Sponsored Content Policy: News-Medical.net publishes articles and related content that may be derived from sources where we have existing commercial relationships, provided such content adds value to the core editorial ethos of News-Medical.Net which is to educate and inform site visitors interested in medical research, science, medical devices and treatments.