The D- and L- system is named after the Latin dexter and laevus, which translates to left and right. The assignment of D and L is used to distinguish between two molecules that relate to each other with respect to reflection; with one molecule being a mirror image of the other. These types of molecules are referred to as chiral for this reason, and the two pairs are called enantiomers.
L and D Isomers. Image Credit: Raimundo79/Shutterstock.com
The stereochemistry of D- and L- enantiomers is defined relative to glyceraldehyde. Glyceraldehyde is selected as the standard to which chiral compounds are compared as chemical manipulation does not affect its configuration, and it has been historically used for this purpose.
D and L isomers are important in pharmacology, as chiral forms of molecules (those of only the L or D isomer for that molecule) have different physiological effects. For this reason, the isomers can now be selectively produced. This allows the delivery of medicines containing chiral molecules in a more targeted, efficacious, and safer way.
Stereochemical classification of optically active molecules
Optically active molecules were discovered in 1843 by Louis Pasteur, who separated crystalline sediments of tartaric acid that accumulated in wine caskets. He discovered that while the crystallized sediments possessed identical shapes and chemical properties, they were mirror images of one another.
The active molecules were also found to rotate light by the same magnitude, but in opposing directions. One type rotated polarized light leftwards (laevorotatory) and the other rightwards (dextrorotatory). X-ray crystallographic studies in 1951 confirmed the ‘handedness’ of tartaric acids and established that the absolute configuration of each.
Stereoisomers were subsequently referred to as molecules that (1) differ in the organization of their atoms in space, (2) retain the same constitution, and (3) are related to one another by a plane of symmetry (are mirror images of one another).
As stereoisomers are present in a pair, they may also be called enantiomers; a term derived from (enántios), meaning 'opposite', and μέρος (méros), meaning 'part'. Furthermore, the ability of these molecules to rotate plane-polarized light by an identical magnitude of opposing directions is referred to as optical activity. The point in the molecule that serves as the anchor for the arrangements of other groups of atoms in space, such that interchanging the positions of any two groups, is called a stereocenter.
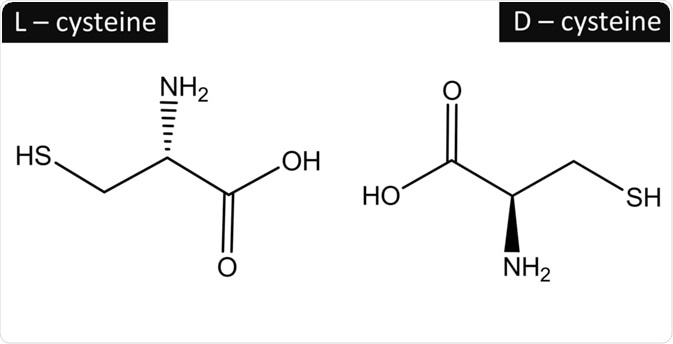
Biologically active molecules are enantiomeric, meaning they possess optical activity. Proteins are one such example of optically active molecules and each enantiomer of the pair are referred to as either L- or D-. The assignment of L- or D- designation is used when representing the molecule in 2 dimensions.
Fischer projections
Fischer projections are a 2D representation of 3D molecules; 3D information can be inferred from the projections; horizontal lines represent groups that project orthogonally out of the plane of paper towards the viewer and vertical lines represent groups that project orthogonally out behind the paper plane. By swapping the groups at the horizontal and vertical positions, enantiomers can be represented.
The stereochemical centers are numbered and this is done according to the priority of the functional groups. Prioritization occurs according to the CORN rule which is an acronym of the groups COOH, R, NH2, and H (where R is the side-chain). The way in which they are arranged around the center of the molecule about which these groups are arranged (called the chiral center). When the hydrogen is positioned behind the plane of view:
- If CO, R, and N groups are arranged in a clockwise fashion about the chiral center, then it the D- isomer
- If the CO, R, and N groups are arranged in an anticlockwise fashion about the chiral center, then it the L- isomer
It is for this reason that the D- and L- designations are used for sugars and amino acids. Furthermore, this type of notation is used specifically for describing them in biological or pharmacological settings where they are prevalent.
The significance of L and D isomers in pharmacology
Stereoisomers differ in the chemical properties of pharmacokinetics and pharmacodynamics. Pharmacokinetic properties refer to how the body affects absorption, distribution metabolism, and excretion.
Absorption
L-methotrexate is better absorbed than D-methotrexate
Distribution
The distribution of S-warfarin within the human body is greater than that of R-warfarin due to its increased ability to bind albumin. Note that the prefixes R and S refer to absolute rotation of plane-polarized light; the R and S designation indicates that the two are enantiomers.
Metabolism
S-warfarin is rapidly metabolized by oxidation to produce 6 hydroxy warfarin and subsequently reduced to warfarin alcohol (R, S), a racemic mixture. R-warfarin, however, is metabolized relatively slower, through the process of side-chain oxidation.
The metabolite produced is 7 hydroxy warfarin and then it is reduced to (S, S) warfarin alcohol. There are also differences in each enantiomer’s half-life: 32 hours for S-warfarin and 54 hours for R-warfarin. This difference is explained by the binding of the enantiomers with misaligned substituents with respect to the chiral center with various binding side pockets.
Pharmacodynamics defines how a particular drug affects the body. Firstly, drug potency varies with respect to stereoisomerism. For example, d-propranolol is inactive whereas the l- form produces the desired beta-blocking action. Secondly, stereoisomerism affects pharmacological effects; this can be seen in methorphan.
The L form is a strong opioid analgesic, whereas the D- form suppresses coughing. Thirdly, stereoisomers differ in their therapeutic ability and adverse effects. For example, D ethambutol is a treatment for tuberculosis, whereas L ethambutol causes blindness. Finally, the stereoisomers can differ in their efficacy, which is defined as how well the desired effect is produced.
These effects on pharmacodynamics and pharmacokinetics illustrate that, usually, one chiral form produces the desired effect in the body whilst the other may cause undesirable or harmful side-effects or an entirely different effect altogether.
As such, there is a need for drugs manufactured in the pharmacological setting to be enantiomerically pure; that is, possessing the desired L or D enantiomer. The advantages of a single enantiomer product are:
- More selective pharmacodynamic products
- An increased therapeutic index, which is a ratio of the amount of a therapeutic drug that produces the desired effect on the amount at which the drug becomes toxic.
- Reduced risk of interaction with other drugs in the body
Stereoisomerism opens new advances in pharmacology, as it aids in refining the efficacy, safety, and potential of existing drugs and allowing exploration of new drug candidates. Several racemic mixtures (mixtures containing both enantiomers) have been replaced by enantiomerically pure drugs that have improved efficacy and reduced toxicity.
Further Reading