Researchers today use electron microscopes for virion detection because of the higher resolution they hold, allowing for images that are 1000 times more magnified than light microscopes. This resolution increase is achieved via a steady beam of electrons (0.005nm wavelength). This is dissimilar to regular light microscopes, which function under the parameters of visible light (380 to 700nm wavelengths).
While most virions have a diameter of 20-400nm, the coronavirus capsid is 100nm in diameter. The electrons that are used in place of photons are directed towards and bounce off the analyte. From here, an artificially colored image can be reconstructed by the sensors fitted to the machine.
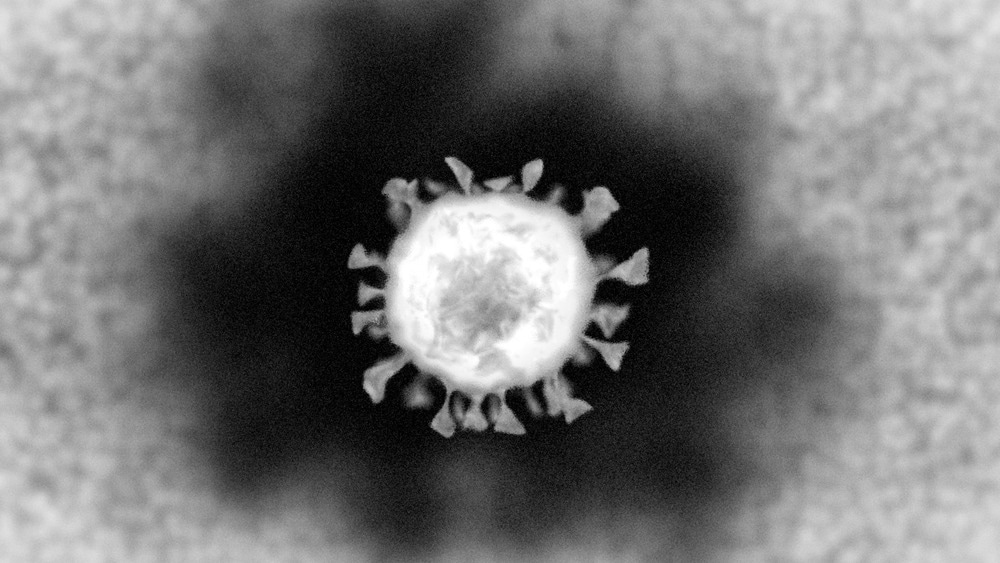
Image Credit: ColinCramm/Shutterstock.com
The Electron Microscope Apparatus
The machine's hull comprises mainly a column for generating electron beams, a specimen chamber, a vacuum pump, and control panels, all fixed to a monitor, which will grant us a visual depiction. Once the specimen chamber has been vented and the pressure is equalized, the analytes (which in this case are cells transfected with SARS-CoV-2) are ready to be assayed.
The column will produce an electron beam that hits the sample from a heated thermionic cathode made of tungsten. Directly below this is the doughnut-shaped anode, and the electric field between the two accelerates the primary electrons to the sample.
An electromagnetic lens and deflection panels found below the anode direct the stream of electrons to the sample of interest. From here, the primary electrons will bounce off the point of incidence and cause the emission of electrons from the sample.
The Raster detector (connected to the deflection panels) is the primary component of directing the electron beam, allowing for the entire surface area of the sample to be measured. Finally, the SE (secondary electron) detector will record the quantity and angle of the emitted electrons from the sample, resulting in the high-resolution images we see in most accredited papers.
How COVID-19 is Prepared for Electron Microscopy
Firstly, the viral vector must be harvested from a patient. This can be accomplished in many ways, though isolation via nasopharyngeal swabs is often the most common. The viral RNA can then be extracted using an appropriate kit, such as the viral RNA mini kits produced by Quiagen or New England Biolabs. The retrovirus undergoes a reverse transcription polymerase chain reaction using the appropriate probes and primers to ensure that the electron microscope surveys a large enough quantity of genomic material.
With the appropriate reagents, these primers will extend to generate cDNA that codes for critical structural proteins that make up the viral capsid, as these proteins are of the greatest interest to researchers. Finally, these viral transcripts will be amplified in cells that the lab's principal investigator determines; these cell lines can vary from Hela cells to HEK 293T cells, Vero cells, and more. After infection occurs at the monolayers of the cell, the plate of cells will be trypsinized, the amount and time of which varies depending on the lab.
The cells are treated with trypsin and EDTA solution so that the cells will detach from whatever plate they are on, as the EDTA chelates calcium and denatures the adhesive forces of the cells. Once titers are calculated and a safe yet substantial amount of virus is generated, electron microscopy can then be applied.
Ultrathin sections of whatever cell is chosen should be obtained for scanning. From here, variability depends on how the principal investigator wishes to proceed with the imaging. Sections are often collected using a copper grid coupled with a high-angle annular dark field detector, which picks up incoherently scattered electrons (Rutherford scattered) rather than directional electron scattering (Brag scattered).
Image Credit: Orpheus FX/Shutterstock.com
What SARS-CoV-2 looks like Under an Electron Microscope
If one were to view lung tissue, myofibrils, or any other site prone to SARS-CoV-2 transfection, one would first see the viral particles trapped within the envelope of certain vesicles. These vesicles/endosomes will appear darker than other matter within the nucleus and cytoplasm of the cell and can be located within the internal membranes of the cells. If the resolution was further increased, one could detect the bristled hexagonal SARS-CoV-2 particles (with roughly 80 nm diameters) that attach to the apical sides of cells.
In previous literature papers, the limitations of this methodology became clear, as it proved difficult to observe the mechanism of particle entry via clathrin-mediated endocytosis. This difficulty is attributed to the small scale on which this mechanism is performed and the speed at which infectious particles bind to the cell's surface.
Recently, however, studies performed by the Oswaldo Cruz institution elucidated the process of Sars-CoV-2 infection into Monkey kidney Vero cells. The lab used electron microscopy to capture just how the virion enters the cell, uses the host's replication machinery, and alters the cellular structure in favor of infectious processes.
Sources:
- Barreto-Vieira, D.F.; da Silva, M.A.N.; de Almeida, A.L.T.; Rasinhas, A.d.C.; Monteiro, M.E.; Miranda, M.D.; Motta, F.C.; Siqueira, M.M.; Girard-Dias, W.; Archanjo, B.S.; et al. SARS-CoV-2: (2022) Ultrastructural Characterization of Morphogenesis in an In Vitro System. Viruses. https://doi.org/10.3390/v14020201
- Caldas, L.A., Carneiro, F.A., Higa, L.M. et al. (2020) Ultrastructural analysis of SARS-CoV-2 interactions with the host cell via high resolution scanning electron microscopy. Sci Rep 10, 16099. https://doi.org/10.1038/s41598-020-73162-5
- Barreto-Vieira DF, da Silva MAN, Garcia CC, Miranda MD, Matos ADR, Caetano BC, Resende PC, Motta FC, Siqueira MM, Girard-Dias W, Archanjo BS, Barth OM. Morphology and morphogenesis of SARS-CoV-2 in Vero-E6 cells. Mem Inst Oswaldo Cruz. 2021 Feb 8;116:e200443. doi: 10.1590/0074-02760200443. PMID: 33566951; PMCID: PMC7874846.
- Brahim Belhaouari D, Fontanini A, Baudoin J-P, Haddad G, Le Bideau M, Bou Khalil JY, Raoult D and La Scola B (2020) The Strengths of Scanning Electron Microscopy in Deciphering SARS-CoV-2 Infectious Cycle. Front. Microbiol. 11:2014. doi: 10.3389/fmicb.2020.02014
- Prasad, Sharda1; Potdar, Varsha4; Cherian, Sarah2; Abraham, Priya†; Basu, Atanu3,* ICMR COVID Team Transmission electron microscopy imaging of SARS-CoV-2, Indian Journal of Medical Research: Feb–Mar 2020 - Volume 151 - Issue 2-3 - p 241-243 doi: 10.4103/ijmr.IJMR_577_20
- Zhang, J., Hwang, J., Isaac, B. et al. (2015) Variable-angle high-angle annular dark-field imaging: application to three-dimensional dopant atom profiling. Sci Rep 5, 12419. https://doi.org/10.1038/srep12419
Further Reading
Last Updated: Nov 8, 2022