G-protein coupled receptors (GPCR) are eukaryotic cell membrane proteins. They are the largest family of transmembrane proteins in mammals, with over 800 different G-protein coupled receptors in humans.
Image Credit: ibreakstock/Shutterstock.com
The prevalence of G-protein coupled receptors underpins how vital they are to signaling within the body. G-protein coupled receptors work by binding specific extracellular ligands, transferring the signal across the membrane, and initiating an intracellular response. Many bodily functions depend on G-protein coupled receptors, with them making up a part of an interconnected web of signaling within the body.
Role in the Body
G-protein coupled receptors have many roles within the body. They transmit signals across the membrane in response to the binding of a specific ligand initiating a cellular signaling cascade resulting in cellular response.
These ligands can vary from hormones to bacterial metabolites. While the downstream response to the binding of these ligands can be considerably complex with significant overlap in the downstream activations caused by the signaling cascades.
In neurons, there are many G-protein coupled receptor families that bind the hormones histamine, cholinergic or somatostatin, or the neurotransmitter adenosine. For instance, the binding of any of these four ligands has the side effect of displaying downstream activation involved with actin dynamics and synaptic transmission, therefore these G-protein coupled receptors are involved in synaptic plasticity and potentially the structural plasticity of neurons.
Not all G-protein coupled receptor ligands are produced by the body. Simple molecules produced by bacteria in the gut have been shown to activate G-protein coupled receptors. These ligands were shown to elicit wide-ranging effects through G-protein coupled receptor binding, including effects on immune recognition, neurotransmission, and inflammation.
Underpinning the diversity in G-protein coupled receptor families and functions are the variations in protein sequence, however, all G-protein coupled receptors share the same molecular structure and are defined as G-protein coupled receptors by this unique conformation.
Molecular Structure of the G-protein Coupled Receptor
With over 800 different G-protein-coupled receptors in humans, there is a wide variation in protein sequence however they all exhibit the same conserved structure. All G-protein coupled receptors have three domains, an extracellular domain containing the N-terminus where the ligand binds, the transmembrane helical domain (TMD) that crosses the membrane seven times, and the intracellular domain containing the C-terminus that activates the signaling proteins.
These proteins create the signaling cascade that results in a cellular response. These signaling proteins can be G-proteins in G-protein dependent signaling, or G-protein-coupled receptor kinase (GRK)-mediated phosphorylation and arrestin coupling in G-protein-independent pathways.
G-protein Coupled Receptor Activation
Activation of receptors results in a conformational change that transfers the signal into the cell. The N-terminus of the G-protein coupled receptors recognizes and binds to the ligand. G-protein coupled receptors only bind ligands that fit the specific N-terminus binding site, different G-protein coupled receptors bind different ligands. These G-protein coupled receptors can be activated by a multitude of different ligands, from photons to proteins.
Upon ligand binding, the activation of the G-protein coupled receptor results in TMD undergoing a conformational change involving a shift of transmembrane domain 6 further away from the rest of the molecule. Thereby resulting in the activation of the dependent molecule through either G-protein dependent signaling or g-protein independent signaling.
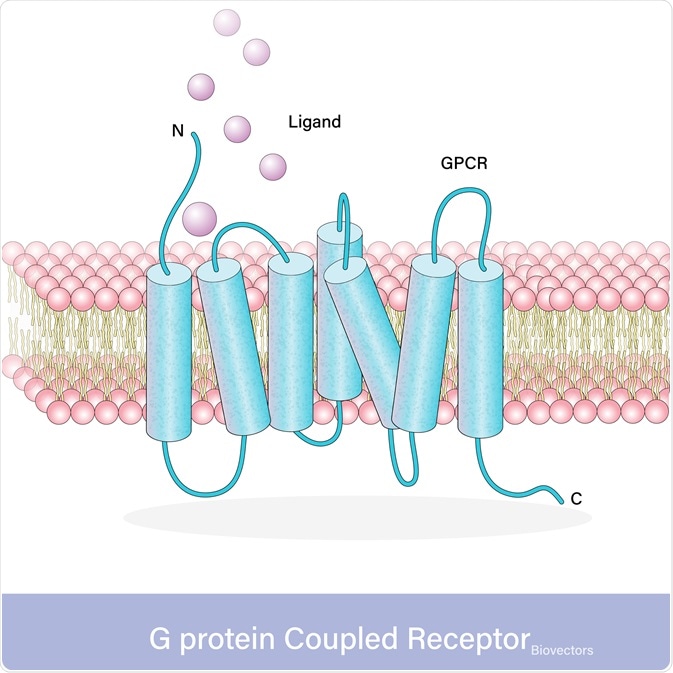
Image Credit: Udayadaithya M V/Shutterstock.com
G-protein Dependent Signalling
The G-protein is heterotrimeric with α, β, and γ subunits. The β and γ units form a dimer that couples with the C-terminus of the G-protein coupled receptor. When inactive this βγ dimer forms a trimer with the α-subunit of the G-protein.
However, the G-protein coupled receptor acts as a guanine nucleotide exchange factor inducing α-subunit exchange the bound GDP for GTP and disassociate from the βγ dimer. This activates α-subunit and the βγ dimer initiating downstream signaling cascades.
Once the GTP is hydrolyzed to GDP within the α-subunit, it reforms an inactive trimer with the βγ dimer, thereby facilitating later activations by the G-protein coupled receptor.
G-protein Independent Signalling
G-protein coupled receptors do not always activate a G-protein, rather the G-protein-coupled receptor kinase (GRK) can phosphorylate the C-terminal domain of the G-protein coupled receptor promoting arrestin coupling. The coupling of arrestin prevents the G-protein coupled receptor from activating the G-protein.
Arrestins contain N-terminus and C-terminus lobes forming a β-stranded sandwich structure which is attached by a hinge region. Through binding to the G-protein coupled receptor, the arrestin undergoes conformal change exposing the phosphointeraction sites of the N-terminus loop. This loop interacts with downstream signaling molecules.
Conclusion
The abundance of G-protein coupled receptors in the body underpins how vital they are to the functioning of the body. They possess a distinctive structure comprised of an extracellular ligand-binding domain, a seven-pass transmembrane helical domain, and an intracellular domain that associates with the G-protein.
G-protein coupled receptors are intermediary signaling molecules, they translate the message across the membrane activating intracellular molecules that initiate a signaling cascade that results in cellular response. These cellular responses can be as diverse as inflammation or synaptic plasticity, while the ligands that initiated them can be as diverse as hormones, photons, or bacterially produced proteins.
This diversity indicates how important the G-protein coupled receptors are to the functioning of many different signaling pathways within the body.
References
- Colosimo, D. A., Kohn, J. A., Luo, P. M., Piscotta, F. J., Han, S. M., Pickard, A. J., Rao, A., Cross, J. R., Cohen, L. J., & Brady, S. F. (2019). Mapping Interactions of Microbial Metabolites with Human G-Protein-Coupled Receptors. Cell Host and Microbe, 26(2), 273-282.e7. https://doi.org/10.1016/j.chom.2019.07.002
- Edward Zhou, X., Melcher, K., & Eric Xu, H. (2019). Structural biology of G protein-coupled receptor signaling complexes. Protein Science, 28, 487–501. https://doi.org/10.1002/pro.3526
- Hilger, D., Masureel, M., & Kobilka, B. K. (2018). Structure and dynamics of GPCR signaling complexes. Nature Structural & Molecular Biology, 25(1), 4–12. https://doi.org/10.1038/s41594-017-0011-7
- Leung, C. C. Y., & Wong, Y. H. (2017). Role of G protein-coupled receptors in the regulation of structural plasticity and cognitive function. Molecules, 22(7), 1–16. https://doi.org/10.3390/molecules22071239
- Manglik, A., & Kruse, A. C. (2017). Structural Basis for G Protein-Coupled Receptor Activation. Biochemistry, 56(42), 5628–5634. https://doi.org/10.1021/acs.biochem.7b00747
- Ribas, C., Penela, P., Murga, C., Salcedo, A., García-Hoz, C., Jurado-Pueyo, M., Aymerich, I., & Mayor, F. (2007). The G protein-coupled receptor kinase (GRK) interactome: role of GRKs in GPCR regulation and signaling. Biochimica et Biophysica Acta - Biomembranes, 1768, 913–922. https://doi.org/10.1016/j.bbamem.2006.09.019
- Syrovatkina, V., Alegre, K. O., Dey, R., & Huang, X.-Y. (2016). Regulation, Signaling, and Physiological Functions of G-proteins. Journal of Molecular Biology, 428(19), 3850–3868. https://doi.org/10.1016/j.jmb.2016.08.002
Further Reading