The term 'proteomics' was initially described by Dr. Marc Wilkins, a Ph.D. student at the time, who defined the scientific word as a "protein complement of a given genome"; this referred to all proteins expressed by cells.
This field has now become a system for analyzing protein populations. The ultimate objective is to establish which protein or protein group is associated with a function or phenotype.
Image Credit: VectorMine/Shutterstock.com
What is Clinical Proteomics?
Clinical proteomics can be described as a comprehensive study of proteins within the body, including qualitative and quantitative profiling. Clinical specimens, including bodily fluids and tissues, can be analyzed from healthy and diseased patients to identify novel biomarkers associated with diseases.
The path towards biomarker discovery can aid as a molecular signature, which can be used to provide a comprehensive understanding of disease stages as the disease progresses within patients. The insight into a patient's disease progression, from early and indolent to late and aggressive stages, can assist physicians with effective treatment management.
This is significant as, with most potentially aggressive diseases, early diagnosis can effectively ensure a patient is treated efficiently, averting adverse symptoms and decreasing morbidity and mortality rates.
Biomarkers can be a useful approach for monitoring patient responses to treatments, ensuring patients are experiencing effective treatments for their corresponding diseases. Ultimately, this can be used to enhance the prognosis rates of patients, as this approach may decipher whether medications are effective for a particular patient, which, if found to be ineffective, can be altered for a more effective treatment.
The field of clinical proteomics can comprise a range of experimental processes, which include well-phenotyped clinical samples and analysis of proteins and peptides that can be used as targets; this can then be used to gather data that is then interpreted and validated for clinical applications.
Biomarker candidates that have been identified through comprehensive profiling have the ability to be translated for clinical applications, which involves a validation study of a cohort of patients. This can be a long process with the aim of deciphering the biomarker's validity for the application of diagnostic or prognostic therapies.
Applications of Clinical Proteomics
This field can be significant for disease identification, including cancer, and can be used to increase cancer therapy, enhancing knowledge of protein markers and the growth of heterogeneous tumors within patients.
DNA microarrays can be used to discover correlations between gene expression and disease subsets, with gene expression profiling allowing for the whole genome study.
An example of this can be found in breast cancer research, with a study finding the expression of genes to be associated with various classes of tumors, including a basal epithelial-like group, ErbB2 overexpressing tumor group, as well as a normal breast group. Studies on these have demonstrated patients belonging to each group have different outcomes, which is a significant piece of information for treatment management, especially for targeted treatments.
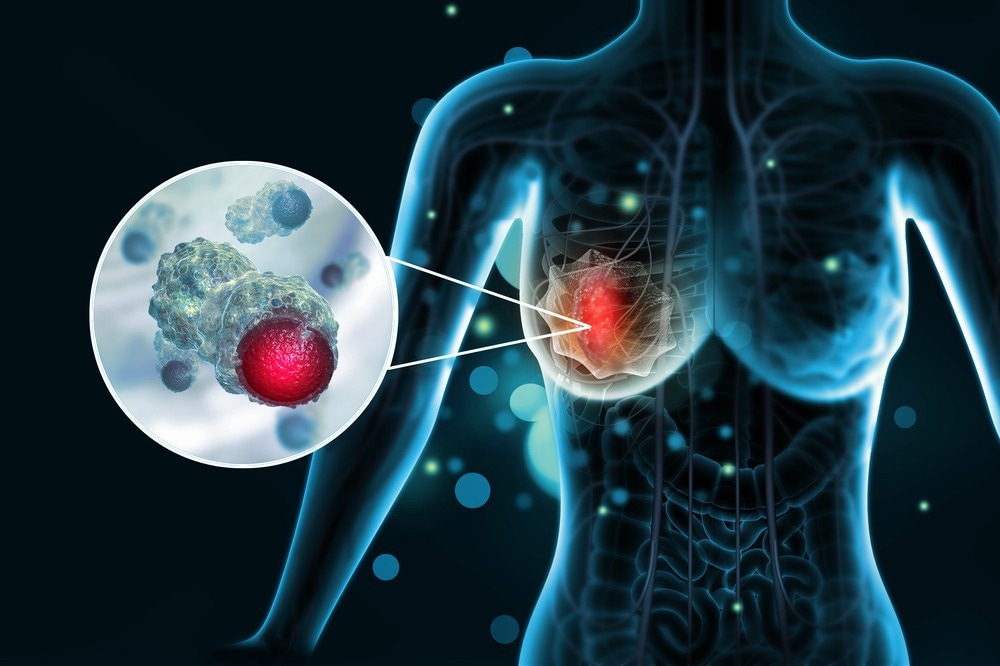
Image Credit: crystal light/Shutterstock.com
Enhancing Treatment
Targeted treatments have been revered in medicine, particularly with the emergence of personalized medicine, due to the notion that diseases are heterogenous and a 'one size fits all' treatment plan may not be very effective for patients.
Patients that experience the same disease may not have the same response when taking the same medication as a treatment; while one patient may have a high response rate for a drug, causing them to be effectively cured, another patient may not have the same effect due to resistance, resulting in the drug to be ineffective.
An example of this is a monoclonal antibody inhibitor of ErbB2, known as trastuzumab, which has been found to be successful in treating metastatic breast cancer in women overexpressing ErbB2 (HER-2).
However, while this drug has been successful, both as a monotherapy and in conjunction with a chemotherapy drug, it was found to have a less than 50% response rate in patients. Additionally, a subset of patients who were responsive to the drug initially was later found to experience disease progression.
The use of microarray analysis has enabled researchers to find drug targets based on other genes modified due to the ErbB2 overexpression, which can be used as part of combination therapy to increase the response rates of resistant patients.
Translational Significance of Clinical Proteomics
Clinical proteomics can aid with identifying drug targets for patients with an array of diseases, including cancer; this can ultimately aid in a comprehensive understanding of how the disease is functioning on a genomic level. Subsequently, this can lead to potentially novel drug candidates that can be studied in clinical trials to enhance the treatment options for patients resistant to conventional drug treatments.
Ultimately, the use of proteomics can assist in personalized medicine to ensure the patient's disease is being wholly comprehended, with related proteins being assessed as potential biological targets.
References:
- BioMed Central. 2022. Clinical Proteomics. [online] Available at: <https://clinicalproteomicsjournal.biomedcentral.com/about> [Accessed 28 May 2022].
- Paik YK., Kim H., Lee EY., Kwon MS., Cho S.Y. (2008) Overview and Introduction to Clinical Proteomics. In: Vlahou A. (eds) Clinical Proteomics. Methods in Molecular Biology™, vol 428. Humana Press. https://doi.org/10.1007/978-1-59745-117-8_1
- Verrills NM. Clinical proteomics: present and future prospects. Clin Biochem Rev. 2006;27(2):99-116. Available at: www.ncbi.nlm.nih.gov/pmc/articles/PMC1579414/
Further Reading