Epifluorescent microscopy is a technique capable of generating images of certain fluorescent samples; however, the sample will appear indistinct if the sample reaches a thickness greater than micrometers. This is because some parts of the sample lie outside of the focal plane.
A new imaging approach was required to overcome this challenge. The confocal approach was driven by Marvin Minsky’s desire to image neural networks in unstained preparations of living brains, and he is widely regarded as the inventor of the confocal microscope in 1955. The original principle was patented in 1957 and is still used in the modern confocal microscope.
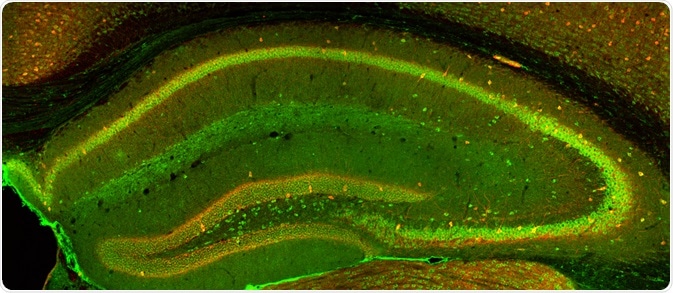
Confocal Imaging. Image Credit: Alexandros A Lavdas/Shutterstock.com
Differences from conventional imaging
In confocal imaging, a focused laser beam is used to produce a small spot illumination on the specimen. This is in contrast to conventional imaging processes, in which the specimen is bathed in excitatory illumination. This modification removes out of focus glare from the final image produced by confocal imaging.
In addition, the shallow depth of field in confocal imaging results in a higher resolution image, which is due to the reduced point spread of the function of the system. This is because the function is the product of the illuminating and objective lenses of the system. The resulting resolution is improved by a theoretical factor of 1.4 in comparison with conventional epifluorescence methods.
How does it work?
The confocal microscope illuminates in a point-by-point fashion and is able to reject out of focus light using a selection mechanism. Laser light with a short wavelength is used to excite the dye in the specimen e.g. a blue laser.
This light is reflected by a dichroic mirror, i.e. a mirror that reflects certain wavelengths of light while allowing others to pass through. The light is then reflected again by two motorized mirrors, which control the scanning of the laser light across the sample.
The fluorescent light excited from the sample (green in the figure above), is then returned to the dichroic mirror by the same motorized mirrors. However, as the light now has a different wavelength, it passes through the dichroic mirror and is then focused onto the pinhole. This pinhole blocks the passage of out of focus light to the detector. The remaining light is then measured by a detector, in this case, a photomultiplier tube (PMT).
It is not possible to capture a complete image of the specimen using confocal microscopy, as this method captures individual points only. For visualization, the detector is connected to a computer that aggregates the individual points to form a complete image of a planar section of the specimen.
Due to the nature of the scanning process, it is only possible to collect light from a single focal plane. By changing the focus of the microscope it is possible to obtain images at different depths. These images can be connected through software to generate a 3-dimensional reconstruction of the sample.
Advantages and limitations
Confocal imaging has a series of advantages when compared to other conventional imaging methods. The main advantages of this method relate to the ability to scan individual layers of a sample.
This relates to the fact that the confocal method collects light from a single focal plane. The ability to change the focal plane means that the user can obtain optical slices at increasing depths within the sample, which can then be combined to produce 3D images.
The small focused laser beam is diffraction limited which means that there is no stray lateral interference, which improves the image contrast. The focused beam also allows removal of the background glow that that would, otherwise, degrade the quality of the image - this is one of the main problems with epifluorescence.
One of the limitations of this method is that the signal strength is reduced by the requirement of a detector pinhole. The reduced signal strength results in the reduced signal to noise ratio. This change in this ratio causes increased sensitivity to noise.
This technique is also more labor-intensive than other imaging processes and therefore requires more training and experience to provide successful images.
Further Reading
Last Updated: Jun 30, 2022