Inflammation is an important stage in the immune system response to damage or invasive foreign bodies and macrophages are involved in the initiation and resolution of it. The response of the immune system includes a wide variety of cells and can be split into five stages, initiation, inflammation, resolution and, tissue‐integrity restoration.
.jpg)
Image Credit: urfin/Shutterstock.com
It is through inflammation that the cells of the immune system treat foreign bodies or damage. Macrophages are vital to the inflammation stage because they can either release pro-inflammatory or anti-inflammatory molecules depending on their polarisation.
This macrophage polarisation is a dynamic process and the balance between the polarised forms affects inflammation and the long-term health of the tissue.
What is polarisation?
Polarisation describes the change in the phenotype of the macrophage. The polarisation of the macrophages results in drastic changes in their global transcriptome. The polarised macrophages are distinguished by molecules they release.
In contrast to differentiation, this polarisation is an easily reversible process. The polarisation of a macrophage occurs in reaction to specific external stimuli. This stimulus encourages polarisation into one of the two main macrophage types, M1 and M2.
M1 Macrophages
M1 macrophages initiate the inflammatory response of the immune system. They become M1 polarised through exposure to lipopolysaccharide or interferon-γ. Interferon-γ is produced by Th1 T-lymphocytes.
These Th1 T-lymphocytes are cells involved with the immune response, they identify and bind to foreign antigens releasing extracellular interferon-γ, consequently this polarises the surrounding macrophages towards the M1 polarisation. This polarisation initiates a cascade of inflammation triggered by the products of M1.
These products contain many pro-inflammatory molecules including cytokines, chemokines, and, reactive oxygen and nitrogen intermediates. These pro-inflammatory species attack the foreign body but lack precision therefore they damage surrounding tissue.
Consequently, inflammation must be carefully controlled which occurs through polarisation to M2 macrophages and their associated anti-inflammatory products.
M2 Macrophages
The resolution of the inflammatory response is dependent on M2 macrophages. They release cytokine mediators, cytokines, and chemokines molecules which act to reduce inflammation. There are four subdivisions of M2 polarised cells, each are proposed to have a distinct anti-inflammatory and tissue repair role.
The M2a polarisation is proposed to be involved in type 2 immunity and allergies, while M2b is a Th2 T-lymphocyte activator and involved in immunoregulation, M2c is also involved in immunoregulation alongside tissue repair, matrix remodeling, and, finally, M2d plays a role in angiogenesis and clearance of apoptotic tissue.
Each of these polarisations is induced by different molecules including Th2, M-CSF, IL-4, IL-10, IL-13. Collectively M2 cells resolve the inflammation response and promote tissue repair.
Sources of Macrophages
The source of the macrophage may affect their initial polarisation. There are two sources of macrophages, those present in the resident tissue or those circulating in the blood. A lot is known about the differentiation of macrophages that originate from the blood.
These macrophages are derived from monocytes under the differentiation factor, macrophage colony stimulation factor (M-CSF). This differentiation factor circulates in high levels in the blood and induces the differentiation of monocytes into M2 macrophages.
Consequently, the natural polarised form for macrophages in the blood is M2. This has important implications on the M1/M2 macrophage balance, thus, inflammation control.
M1/M2 Balance
The balance between the M1/M2 macrophage polarisations controls the duration and severity of the inflammation reaction. While the M1 cells induce inflammation the M2 macrophages resolve inflammation therefore the balance between the two polarisations is critical to the restoration and maintenance of healthy tissue.
The ratio of M1: M2 macrophages is dynamic, with cells switching between the two polarisations according to outside stimuli. It is believed that the M2 polarisation is the natural state of macrophages with them polarising to M1 when induced by T-lymphocytes producing interferon-γ or lipopolysaccharides.
These two pressures for M1 polarisation are responsive, they occur in reaction to infection or damage, once the stimulus is removed then the M1/M2 balance should be restored. Thereby allowing the M2 polarised cells to reduce inflammation and restore the tissue.
The duration of the inflammation can vary from acute, lasting minutes or hours, to chronic, and lasting months or years. If the source of the inflammation cannot be resolved and macrophages remain M1 polarised, then the M1/M2 will remain imbalanced resulting in tissue damage.
In response to allergens, the M1/M2 imbalance is towards the M2 polarisation. These M1/M2 imbalances have been linked to conditions such as asthma, chronic obstructive pulmonary disease, atherosclerosis, or osteoclastogenesis in rheumatoid arthritis patients.
In cancer, the macrophages have been seen to be M2 dominant in lung tumors but M1 dominant in colon carcinomas. Consequently, the balance of M1/M2 polarisations is crucial to the maintenance of healthy tissue.
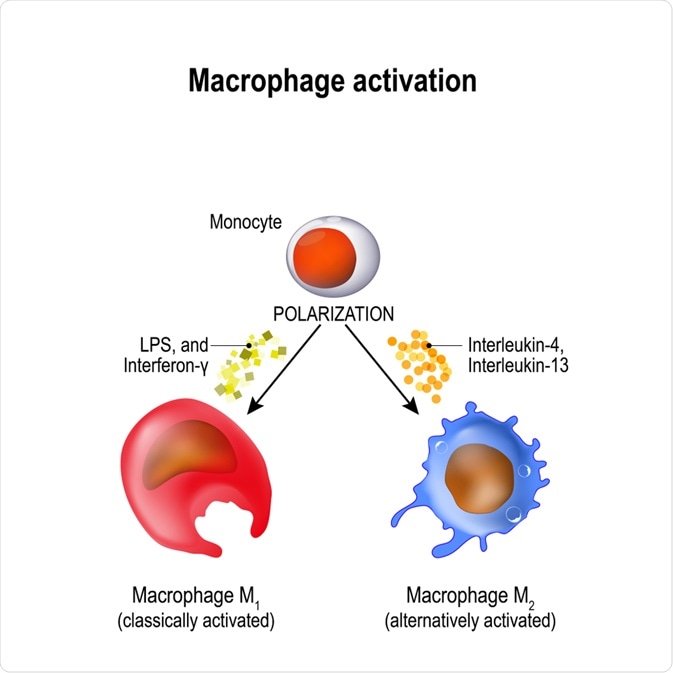
Image Credit: Designua/Shutterstock.com
Summary
Macrophages play an important role in the immune system. They have a key role in inducing and resolving inflammation. The role the macrophage plays is dependent on the polarisation of the cell. M1 polarised cells induce inflammation whereas M2 polarised cells resolve it.
Polarisation refers to the global change in the transcriptome thus the molecules that the cell produces. The polarisation of macrophages is a dynamic process and occurs through external stimuli.
This way, the macrophages adapt to the needs of the surrounding tissue whether that be inducing inflammation or reducing inflammation and promoting healing.
References
- Artyomov, M., Sergushichev, A. and Schilling, J. D. (2016) ‘Integrating Immunometabolism and Macrophage Diversity’, Seminars in Immunology, 28(5), pp. 417–424. doi: 10.1016/j.smim.2016.10.004.
- Atri, C., Guerfali, F. Z. and Laouini, D. (2018) ‘Role of human macrophage polarization in inflammation during infectious diseases’, International Journal of Molecular Sciences, 19. doi: 10.3390/ijms19061801.
- Funes, S. C. et al. (2018) ‘Implications of macrophage polarization in autoimmunity’, Immunology, 154(2), pp. 186–195. doi: 10.1111/imm.12910.
- Martinez, F. O. et al. (2006) ‘Transcriptional Profiling of the Human Monocyte-to-Macrophage Differentiation and Polarization: New Molecules and Patterns of Gene Expression’, The Journal of Immunology, 177(10), pp. 7303–7311. doi: 10.4049/jimmunol.177.10.7303.
Further Reading
Last Updated: Jun 17, 2020