The major histocompatibility complexes (MHC) are cell surface glycoproteins whose primary functions are to present peptide fragments for recognition by the appropriate T cells (lymphocytes). Therefore, the immune system will be able to recognize and kill foreign substances. MHC, which is also called the human leukocyte antigen (HLA) system, is located on the short arm of chromosome number 6 as a highly polymorphic region in the human genome.
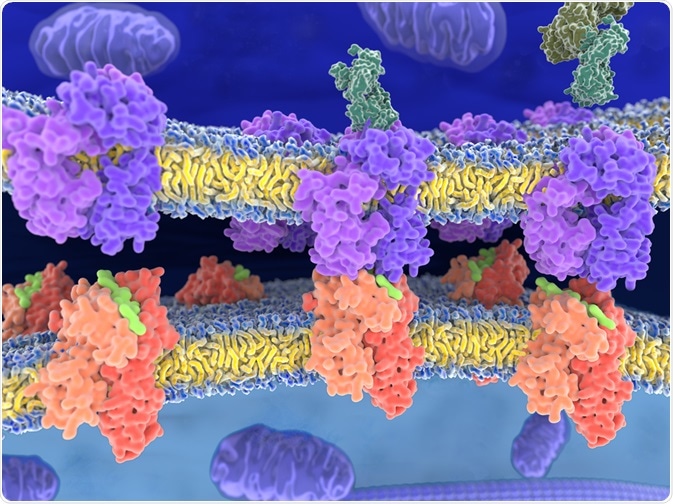
Image Credit: Juan Gaertner/Shutterstock.com
Types of MHC
Class I and class II are the two main types of MHC protein molecules. Class III molecules include other immune proteins that are not related to antigen presentation. MHC class I and II proteins have an important role in the adaptive immune system. Both of them have the common task of presenting peptides to be recognized by T cells on the cell surface.
Although almost all nucleated cells express MHC class I molecules, MHC class II molecules are restricted to specific cells called antigen-presenting cells (APCs), including B cells, macrophages, and dendritic cells. The presentation of peptides by MHC class I molecules on nucleated cells are recognized by cytotoxic CD8+ T cells, whereas MHC class II on APCs can activate CD4+ T cells, resulting in successful regulation and coordination effector cells.
MHC class I molecules presented cytosolic proteins, which are mainly intracellular proteins. They are mainly self-peptides, which are derived from defective ribosomal products and protein turnover. These cytosolic proteins are degraded in the proteosomes and presented by MHC class I molecules during cancerous transformation, intracellular microorganism infection, or viral infection.
The presentation of degraded proteins by MHC class I molecules to CD8+ T cells induces the release of perforin from CD8+ T cells to form pores into the target cell membrane. MHC II molecules, unlike MHC I molecules, are meant to present extracellular pathogens rather than intracellular. Phagocytosis of pathogens is usually the first step in this process. Lysosomes secrete lysosomal enzymes, which break down pathogens inside the cells. Specific peptides are presented by MHC II molecules to CD4+ T cells, leading to activation of CD4+ T cells. Then, cytokines will be released from activated CD4+ T cells, helping in inducing other cells and combating the pathogens outside the cells.
Class I MHC molecule consists of two heterodimer polypeptide chains, α, and β2-microglobulin that are noncovalently linked together via interaction of beta-2 microglobulin with α3 domain of alpha chain. Although class II MHC molecules are also heterodimers, they have two homogenous peptides (α and β polypeptide chain) both of which are encoded in the MHC.
The α chain consists of two domes (α1 and α2). The β polypeptide chain has two domains (β1and β2). Antigen binding domains are α1and α2 domains in class I MHC molecule, and α1 and β1 domains in class II MHC molecules.
Role of the MHC in autoimmunity
In 1967, the MHC was first correlated to disease when HLA-B antigens were found in Hodgkin's lymphoma patients at increased frequency. Then, the majority of autoimmune diseases, as well as many inflammatory and infectious diseases are associated with variation within the MHC.
Several conditions including multiple sclerosis, Crohn’s disease, and rheumatoid arthritis are associated with specific MHC molecules. Polymorphisms of MHC complexes could change the outcome of discouraging T cells from brewing autoimmunity, with preventive MHC purging deleterious clonotypes and disease-promoting MHC encouraging their escape.
A pooled analysis of MHC disease associations reported that there is shared disease susceptibility to alleles that originate from HLA-DR4 haplotypes. Hence, there is a common, as well as disease-specific, association between MHC complexes and autoimmunity.
Until now, there is no definitive mechanism to clearly explain the relationship between MHC complexes and autoimmune diseases, but it may be due to an alteration of tolerance to self-antigens in aberrant MHC class II molecule antigen presentation. Thus, specific MHC class II alleles could be determinates of autoantigen targeting, leading to the association of MHC complexes and autoimmune diseases.
Allorecognition and MHC
If T cells recognize and react to allogeneic (foreign) MHC molecules, allorecognition occurs. Direct, indirect, and semi-direct are the three main pathways of allorecognition. T cells recognize unprocessed allogeneic MHC molecules on graft APCs in direct allorecognition, whereas in indirect allorecognition, there will be a presentation of processed peptide of allogenic MHC molecules bound to self MHC molecule.
The uptake and surface expression of intact foreign MHC+ peptide complexes via recipient APCs occur in the semi-direct pathway, allowing for both indirect and direct recognition of foreign MHC on the surface of the same APCs by the recipient T-cells. Direct, indirect, and semi-direct pathways could contribute differentially to the alloresponse over time.
Sources:
- Janeway Jr, Charles A., et al. "The complement system and innate immunity." Immunobiology: The Immune System in Health and Disease. 5th edition. Garland Science, 2001. https://www.ncbi.nlm.nih.gov/books/NBK10757/
- Mahdi, Batool Mutar. "Introductory chapter: concept of human leukocyte antigen (HLA)." Human leukocyte antigen (HLA). IntechOpen (2019): 1-8. https://www.intechopen.com/chapters/65210 DOI: 10.5772/intechopen.83727
- Wieczorek, Marek, et al. "Major histocompatibility complex (MHC) class I and MHC class II proteins: conformational plasticity in antigen presentation." Frontiers in immunology 8 (2017): 292. https://doi.org/10.3389/fimmu.2017.00292
- Tsai, Sue, and Pere Santamaria. "MHC class II polymorphisms, autoreactive T-cells, and autoimmunity." Frontiers in immunology 4 (2013): 321. https://doi.org/10.3389/fimmu.2013.00321
Further Reading
Last Updated: Nov 30, 2021