Synapses are minute cell projections through which electrochemical impulses are transferred between nerves.
Extended periods of stress in the brain leads to withdrawal of synapses and maladaptive changes to circuits associated with the development of major depressive disorder.
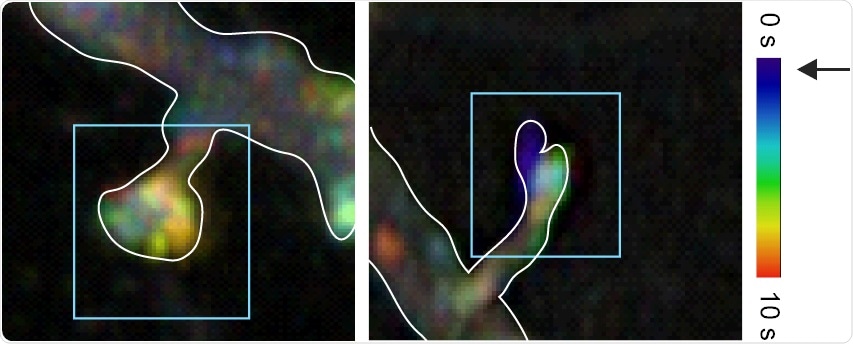
Blocking activation of the JNK protein in synapses stops synapses from retracting. Temporal color-coding shows that when the JNK protein is inhibited in synapses using a light beam, a structural protein called “actin” freezes in time. Changing the cell’s cytoskeleton is part of an array of molecular events that JNK triggers to destabilize synapses. Image Credit: University of Turku
To turn off the activity of a protein known as JNK particularly in synapses, Postdoctoral Researcher Patrik Hollós and his team made use of a light-activated optogenetic tool.
According to Hollos, shrinkage of synapses due to stress can be prevented by using a light beam to block the JNK proteins. He added that the internalization of the “AMPAR” receptor—an early event in the disassembly of synapses—was specifically blocked.
JNK is a stress sensor in synapses and may elicit the effects of ketamine
Scientists identified that ketamine—a novel, fast-acting anti-depressant compound—blocks the JNK protein and prevents synapse retraction.
These outcomes reveal that the JNK protein acts as a stress sensor in synapses. Upon activation, it initiates the disassembly of synapse machinery, which eventually leads to rapid synapse regression.
By contrast, when the JNK protein is inhibited, the synapses can withstand chronic endocrine stress. This may be applicable for conditions in which hormonal stress causes elimination of synapses and control of synapse number under normal homeostatic conditions, stated Eleanor team leader.
The study outcomes help researchers gain insights into how stress disassembles synapses, providing evidence to develop novel targeted therapies.
Source:
Journal reference:
Hollos, P., et al. (2020) Optogenetic Control of Spine-Head JNK Reveals a Role in Dendritic Spine Regression. eNeuro. doi.org/10.1523/ENEURO.0303-19.2019.